Geology 3 (An Overview of Atoms and Chemical Bonds)
TLDRThe script provides an overview of chemistry and matter. It explains that matter is anything that has mass and takes up space, including all physical objects. Atoms, the building blocks of matter, are composed of protons, neutrons, and electrons. Atoms bond together to form molecules and compounds. The script also covers concepts like radioisotopes, half-lives, pH scales, and how water exhibits unique properties due to its polar molecular structure. Overall, the script aims to build an understanding of matter at the atomic and molecular level to explain macroscopic chemical properties and phenomena.
Takeaways
- 😀 Matter is anything that has mass and takes up space, including us, objects around us, and even interstellar dust and asteroids.
- 🔬 Chemistry studies types of matter and how they interact with each other through chemical reactions and transformations.
- 🌱 Matter gets recycled through nutrient cycles and ecosystems, so pollution and waste don't simply disappear.
- 🛰 Most elements originated from dying stars, exploding stars, merging neutron stars or the Big Bang, so we are made of stardust.
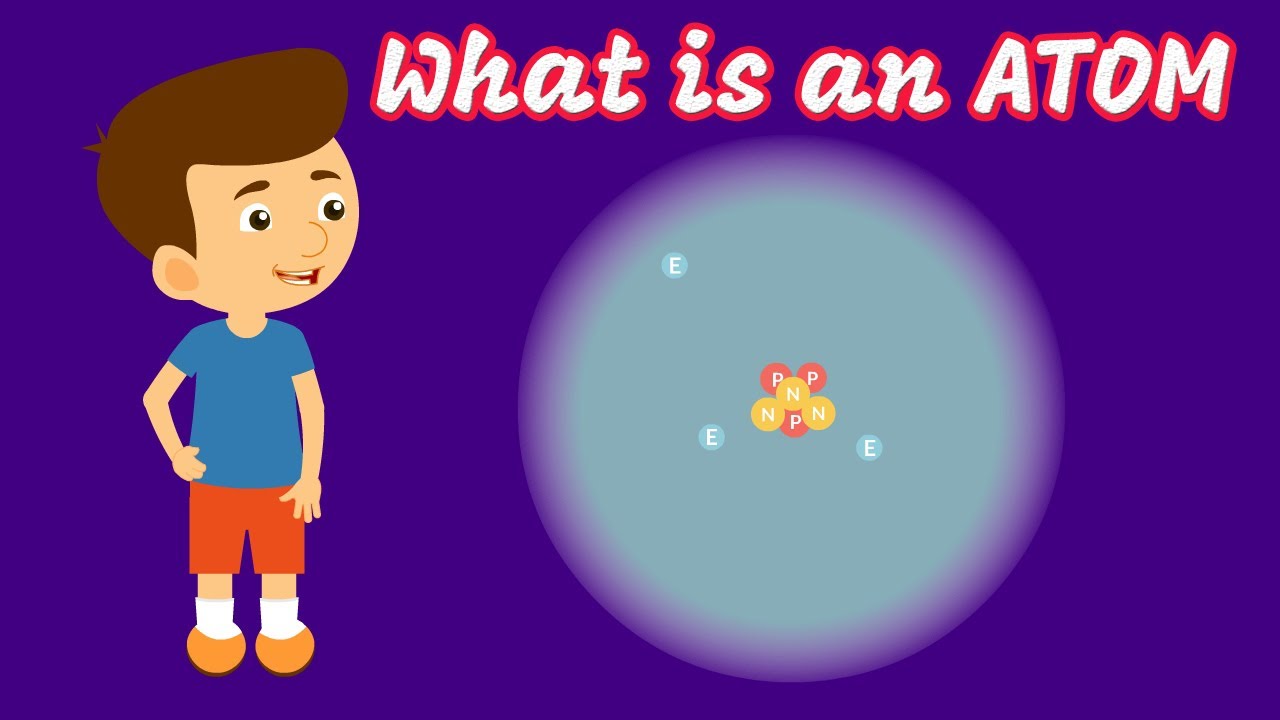
- ⚛️ Atoms have a nucleus of protons and neutrons, surrounded by an electron cloud in various shells and orbitals.
- 🤝 Atoms form bonds and combine into molecules and compounds by sharing, transferring or attracting electrons.
- 💧 Water is a polar molecule that enables transportation of nutrients, stabilizes temperature, and dissolves vital substances.
- 🔋 pH measures acidity and ranges from 0-14, with lower values being more acidic, 7 neutral and higher values more basic.
- 📊 The pH scale is logarithmic, so a 1 unit change reflects a 10x difference in hydrogen ion concentration.
- ⏳ Radioactive isotopes decay over time according to their unique half-lives until they become stable non-radioactive isotopes.
Q & A
What is the definition of matter?
-Matter is defined as all material in the universe that has mass and occupies space, including everything around us like tables, air, water, interstellar dust, etc.
What is the law of conservation of matter?
-The law states that matter can transform from one type of substance into another, but it cannot be created or destroyed. The total amount of matter stays constant.
What are atoms made up of?
-Atoms consist of a nucleus containing protons and neutrons, surrounded by an electron cloud or shells. The protons are positively charged, neutrons have no charge, and electrons are negatively charged.
What causes atoms to form molecules and compounds?
-Atoms bond together due to the attraction between their electrons and nuclei. They form molecules when two or more atoms combine, and compounds when atoms of different elements combine.
Why is water considered a polar molecule?
-Water has an unequal sharing of electrons between the oxygen and hydrogen atoms. This makes the oxygen end slightly negative and hydrogen end slightly positive, giving water a polarity.
What does the pH scale measure?
-The pH scale measures the acidity or alkalinity of a solution by quantifying the concentration of hydrogen ions (H+) present. Solutions with more hydrogen ions are more acidic.
What are some everyday items that are acidic or basic?
-Some acidic items are orange juice, vinegar, soda. Basic items include baking soda solution, seawater, bleach, and ammonia.
Why is water less dense as ice than liquid water?
-This unique property of water causes ice to float at the surface rather than sink. This insulates the water below and protects aquatic ecosystems in winter.
What are isotopes?
-Isotopes are varieties of the same element with different numbers of neutrons. Isotopes can be stable or radioactive.
How does radioactivity relate to half-life?
-Radioactive isotopes decay over time. Half-life is the time it takes for half the atoms of a radioactive isotope to decay.
Outlines
😀 What is Chemistry and Matter
This paragraph introduces chemistry as the study of matter and energy. It defines matter as anything with mass that occupies space, giving examples like people, tables, air, etc. It mentions the law of conservation of matter, which states that matter can change forms but not be created or destroyed. It notes that pollution and waste cannot simply disappear due to this law.
🌟 Atoms and Their Structure
This paragraph discusses the fundamental components of matter - atoms. It explains that atoms contain a positively charged nucleus made up of protons and neutral neutrons, surrounded by a negatively charged electron cloud. It introduces important atomic concepts like atomic number, atomic mass, isotopes, ions, the periodic table and radioisotopes.
📊 The Periodic Table Shows Origins of Elements
This paragraph talks about the periodic table, noting how it lays out elements by atomic number and structure. It discusses a colorful periodic table that shows the cosmic origins of different elements. It explains how dying stars, exploding stars, merging neutron stars etc. led to creation of many naturally occurring elements.
🔋 Ions and Chemical Bonding Between Atoms
This paragraph discusses how atoms can gain/lose electrons to become positively/negatively charged ions. It explains chemical bonding - how atoms bind by attraction to electrons, either sharing equally (covalent bond) or transferring completely (ionic bond). It distinguishes between molecules and compounds and gives examples of each.
💧 Unique Properties of Water Molecules
This paragraph talks about the polarity and hydrogen bonding between water molecules which gives rise to cohesion and surface tension. It explains how water can absorb a lot of heat energy before changing temperature, leading to climate stabilization. It also notes water's unusual property of ice floating above liquid water due to the density difference.
😮 Acidity, Basicity and the pH scale
This final paragraph introduces the pH scale as a quantitative measure of acidity and hydrogen ion concentration in solutions. It notes that acidic solutions have pH below 7 and basic ones above 7. It lists the pH values of various common substances and explains the logarithmic nature of the scale.
Mindmap
Keywords
💡Matter
💡Atom
💡Ion
💡Isotope
💡pH
💡Electron
💡Molecule
💡Compound
💡Covalent bond
💡Hydrogen bond
Highlights
Chemistry studies types of matter and how they interact with one another.
Matter is anything that has mass and occupies space, including all material in the universe.
The law of conservation of matter states that matter can transform but cannot be created or destroyed.
Elements are fundamental types of matter defined by their chemical properties and composed of atoms.
Atoms contain a nucleus of protons and neutrons, surrounded by an electron cloud in orbitals.
Isotopes are atoms of the same element with different numbers of neutrons that can behave differently.
The periodic table organizes elements by atomic number and shows the cosmic origins of atoms.
Radioactive isotopes decay by emitting radiation over a characteristic half-life until stable.
Ions are charged atoms that gain/lose electrons, facilitating chemical bonding between elements.
Molecules are combinations of atoms, while compounds contain multiple elements.
Water has many unique properties due to unequal sharing of electrons in hydrogen bonds.
The pH scale from 0-14 measures a solution's acidity by its concentration of hydrogen ions.
Atoms bond together due to attraction between their electrons, either sharing or transferring them.
Covalent bonds involve sharing electrons, while ionic bonds completely transfer electrons.
A small change in pH reflects a large difference in chemistry due to the logarithmic scale.
Transcripts
Browse More Related Video

Basic Atomic Structure: A Look Inside the Atom

What is Chemistry? | Science for Kids | Chemistry for Kids | STEM for Kids

GENERAL CHEMISTRY explained in 19 minutes

Atoms and Matter for Kids

What Is An Atom - Part 1 | Properties of Matter | Chemistry | FuseSchool
What is an Atom? - Structure of an Atom - Atom video for kids
5.0 / 5 (0 votes)
Thanks for rating: