What is Chemistry? | Science for Kids | Chemistry for Kids | STEM for Kids
TLDRThis educational video introduces the fundamental concepts of chemistry, a branch of science that explores the properties of matter. It explains that everything in the universe is composed of atoms, which are the building blocks of all substances. The video delves into the structure of atoms, including the nucleus with protons and neutrons, and the orbiting electrons. It highlights the significance of the periodic table in categorizing elements by the number of protons. The script also touches on molecules, using water (H2O) as an example, and emphasizes chemistry's role as the central science that connects various fields like physics, math, biology, and medicine.
Takeaways
- 🧪 Chemistry is a branch of science that studies the properties of matter and everything in the universe is made up of matter.
- 🌌 Matter is composed of tiny particles called atoms, which are the building blocks for everything.
- 🪨 Atoms can combine in different ways to create different substances, like comparing a rock and a banana both made of atoms but with different properties.
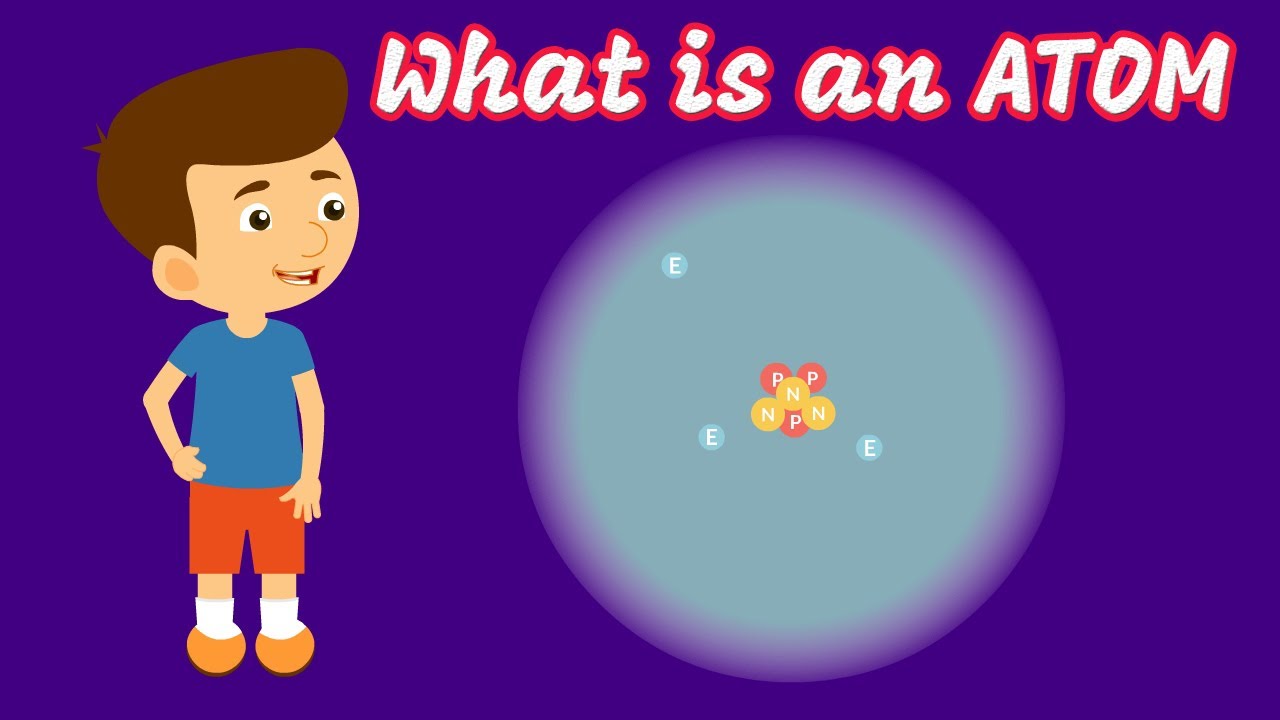
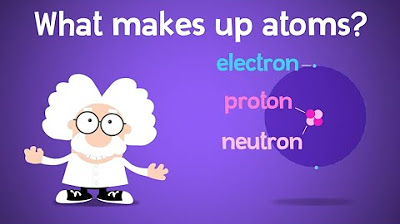
- 🔬 An atom has a central core called a nucleus, made up of protons and neutrons, with electrons orbiting around it.
- ☀️ The nucleus of an atom is analogous to the sun, with electrons as planets orbiting around it.
- 🔍 The number of protons in an atom's nucleus determines the type of element it is, such as hydrogen with one proton or helium with two.
- 📊 The periodic table lists all chemical elements in the world, ordered by the number of protons in their nuclei.
- 💧 A molecule is formed when two or more atoms combine, with water (H2O) being a common example consisting of two hydrogen atoms and one oxygen atom.
- 🔬 Chemists study atoms and molecules to understand the physical properties of substances and how everything in the world works.
- 🔄 Chemistry is often called the central science because it connects various fields like physics, math, biology, and medicine.
- 📚 The script encourages further learning about chemistry and related topics such as the states of matter and gravity.
Q & A
What is chemistry?
-Chemistry is a major branch of science that studies the properties of matter and how atoms interact and bond together to form different substances.
What are the basic building blocks of everything in the universe?
-Atoms are the basic building blocks of everything in the universe, including buildings, plants, humans, animals, and everything in between.
How do atoms determine the properties of an object?
-The way atoms interact with each other and are bonded together can dictate the shape, color, texture, and other properties of an object.
What is the central core of an atom called?
-The central core of an atom is called the nucleus, which is made up of protons and neutrons.
What are the negatively charged particles that spin around the nucleus of an atom?
-Electrons are the negatively charged particles that spin around the nucleus of an atom, similar to planets orbiting the sun.
What makes one atom different from another?
-The number of protons in the nucleus of an atom is what makes one atom different from another, determining its identity as a specific element.
What is the significance of the periodic table in chemistry?
-The periodic table lists all of the chemical elements in the world in the order of how many protons each nucleus has, providing a systematic way to understand and categorize elements.
What is a molecule, and how is it formed?
-A molecule is a group of two or more atoms that form and combine together. It is created when atoms share or exchange electrons to achieve a stable electron configuration.
What is the chemical formula for water, and what does it represent?
-The chemical formula for water is H2O, representing a molecule that consists of two hydrogen atoms and one oxygen atom.
Why is chemistry often referred to as the central science?
-Chemistry is often referred to as the central science because it integrates and connects various other fields such as physics, math, biology, medicine, and more, providing a foundation for understanding the world.
What can viewers expect from the next STEM and Rabbit lesson?
-In the next STEM and Rabbit lesson, viewers can expect to learn about gravity, with more stimulating lessons and activities to enhance their understanding of science.
Outlines
🌌 Introduction to Chemistry
The script introduces the audience to the field of chemistry, a major branch of science that studies the properties of matter. It explains that everything in the universe is made up of matter, which in turn is composed of atoms. The script uses the analogy of a rock and a banana to illustrate how different objects are made of the same fundamental particles, atoms, but differ in their properties due to the way these atoms interact and bond. The concept of atoms having a nucleus with protons and neutrons, and electrons orbiting the nucleus, is introduced. The script also touches on the uniqueness of atoms based on the number of protons in their nucleus, which defines the element they represent, such as hydrogen or helium.
📚 The Periodic Table and Elements
This section of the script delves into the periodic table, which is a catalog of all chemical elements organized by the number of protons in their atomic nuclei. It explains how the number of protons determines the identity of an element, and how these elements are the building blocks of matter. The script also mentions molecules, which are formed when two or more atoms combine, using water (H2O) as an example to explain the composition of molecules. The periodic table is presented as a tool that helps in understanding the properties and interactions of elements.
🔬 Chemistry as the Central Science
The script concludes by emphasizing the importance of chemistry as the 'central science,' highlighting its interdisciplinary nature and its connections to physics, math, biology, medicine, and more. It underscores the role of chemistry in understanding the world and its phenomena. The video encourages viewers to continue exploring chemistry and related sciences, mentioning upcoming lessons on the states of matter and gravity, and inviting the audience to engage with the content through likes, comments, and subscriptions.
Mindmap
Keywords
💡Chemistry
💡Atoms
💡Nucleus
💡Electrons
💡Protons
💡Neutrons
💡Elements
💡Periodic Table
💡Molecules
💡Chemical Bonds
💡Central Science
Highlights
Introduction to chemistry as a branch of science that studies the properties of matter.
Matter in the universe is composed of tiny particles called atoms.
Atoms are the building blocks for everything in the universe.
Comparison of a rock and a banana to illustrate that both are made up of atoms.
Explanation of an atom's structure with a nucleus and orbiting electrons.
The nucleus contains protons and neutrons, while electrons have energy and are negatively charged.
Protons in the nucleus determine the type of element an atom becomes.
Introduction of the periodic table listing chemical elements by the number of protons.
Formation of molecules when two or more atoms combine, exemplified by water (H2O).
Chemistry's role in understanding the physical properties of substances.
Chemistry as the central science, connecting various fields such as physics, math, biology, and medicine.
Invitation to explore more about chemistry and its applications in the world.
Teaser for the next STEM lesson on the states of matter.
Upcoming STEM and rabbit lesson on gravity.
Encouragement to like, comment, and subscribe for more educational content.
The transcript ends with a musical note, indicating the conclusion of the lesson.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: