What is Density? | Relative Density | Floatation
TLDRThis educational video explores the concepts of density, relative density, and flotation through a hands-on experiment. It demonstrates how liquids with different densities like honey, water, and kerosene form distinct layers and affect the buoyancy of various objects. The video explains the formula for density, its units, and introduces the concept of relative density, or specific gravity. It also discusses the principles behind why objects float or sink in liquids and poses a thought-provoking question about the buoyancy of steel ships, encouraging viewers to engage in the comment section.
Takeaways
- 🍯 The experiment demonstrates the concept of density and flotation using honey, water, and kerosene to create distinct layers.
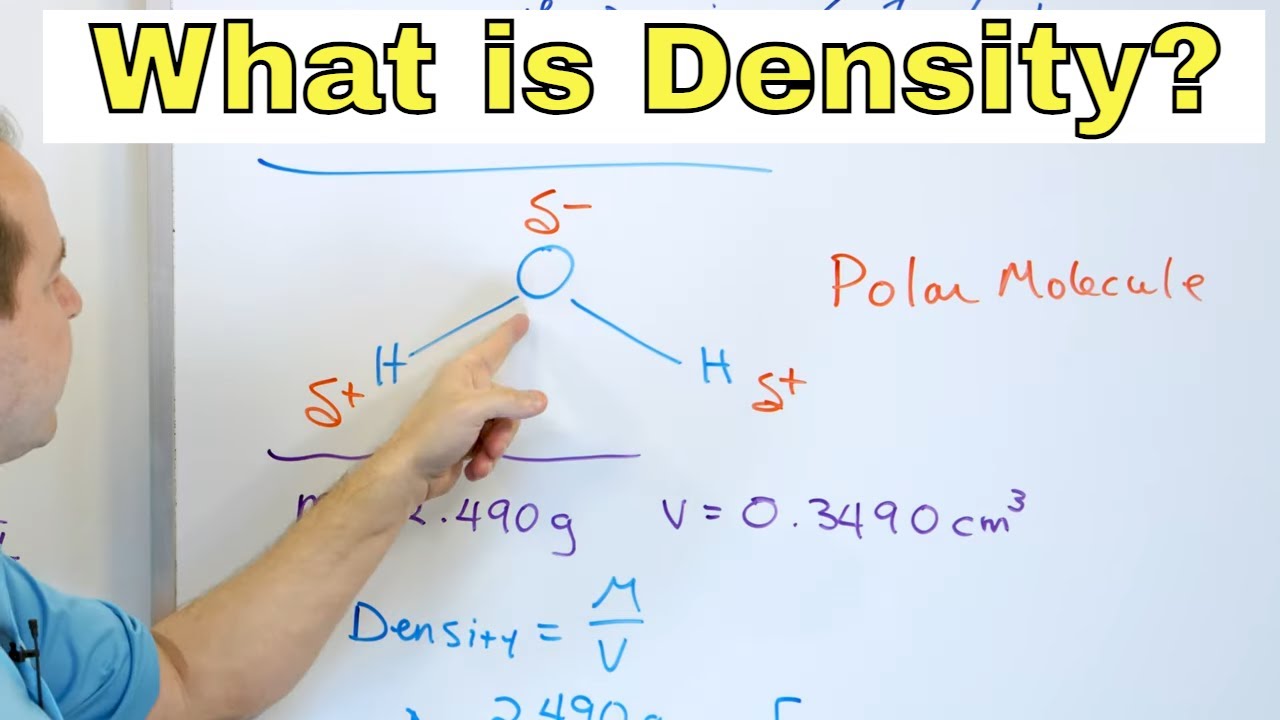
- 📚 Density is defined as mass per unit volume and is symbolized by the Greek letter rho (ρ), with the formula ρ = M/V.
- 📏 The SI unit of density is kilograms per cubic meter (kg/m³), while the CGS unit is grams per cubic centimeter (g/cm³).
- 💧 A unique property of water is that its density is 1 g/cm³ in CGS units, making it a baseline for understanding relative densities.
- 🔄 The relative density, or specific gravity, of a substance is its density compared to that of water, and it is unitless.
- ⛔ The steel nail sinks in all liquids because its density is greater than the densities of honey, water, and kerosene.
- 🍇 The grape floats on honey but sinks in water, indicating its density is between that of water and honey.
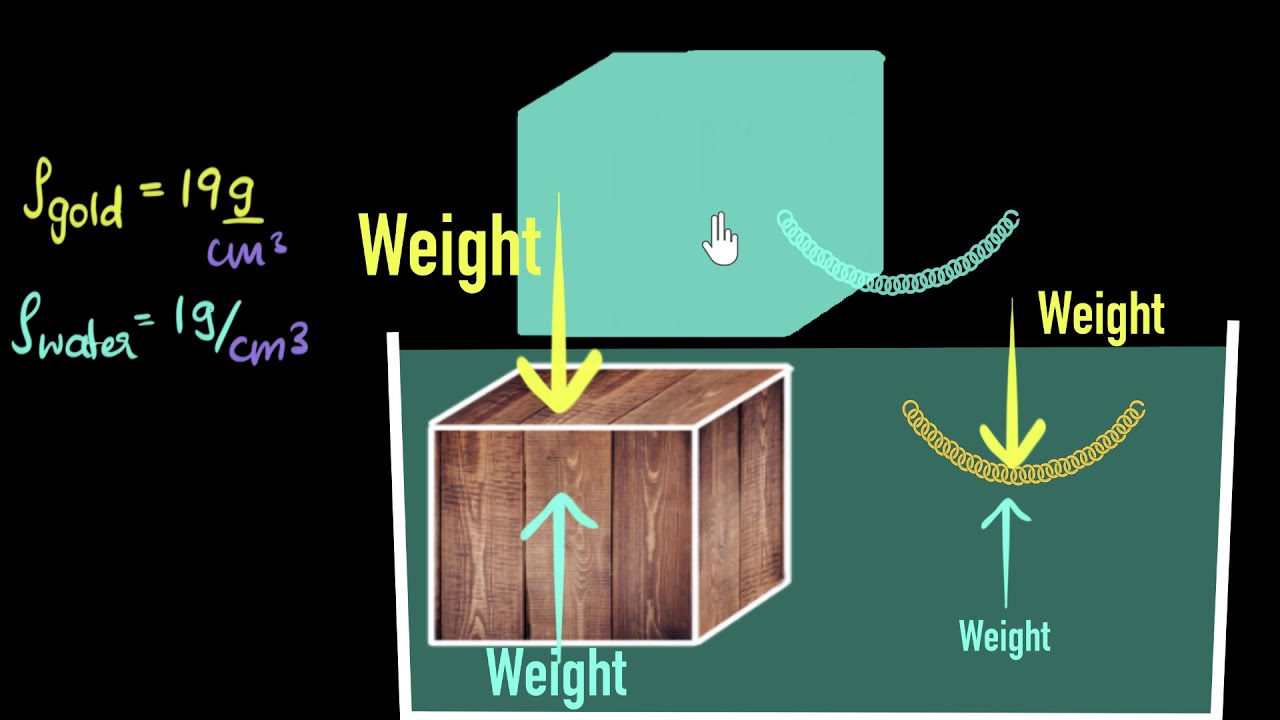
- 🚢 Despite being made of steel, a ship can float due to its design, which displaces a volume of water with a weight equal to or greater than the ship's weight.
- 🏺 The bottle cap's behavior suggests its density is between that of kerosene and water, sinking in kerosene but floating in water.
- 🎾 The plastic ball floats on kerosene, indicating its density is less than that of kerosene.
- 🧊 An ice cube sinks in kerosene but floats in water, showing its density is between that of water and kerosene.
Q & A
What is the main concept discussed in the video script?
-The main concept discussed in the video script is the concept of density and its relation to the relative density and flotation of objects in liquids.
What experiment is conducted in the video to demonstrate the concept of density?
-The experiment involves pouring honey, water, and kerosene into a glass to create three distinct layers and then dropping various objects to observe their behavior in these liquids based on their densities.
Why does kerosene float on water and honey sink below water?
-Kerosene floats on water because its density is less than that of water. Similarly, honey sinks below water because its density is greater than that of water.
What is the formula for calculating density?
-The formula for calculating density is density (ρ) equals mass (M) divided by volume (V), or ρ = M/V.
What are the SI and CGS units of density?
-The SI unit of density is kilograms per meter cube (kg/m³), and the CGS unit is grams per centimeter cube (g/cm³).
Why does the density of water have a value of one gram per centimeter cube in CGS units?
-The density of water is one gram per centimeter cube in CGS units because by definition, one cubic centimeter of water has a mass of one gram.
What is the conversion between CGS and SI units of density?
-The conversion between CGS and SI units of density is that one gram per centimeter cube is equal to 1000 kilograms per meter cube.
What is meant by relative density, and what is its unit?
-Relative density, also known as specific gravity, is the ratio of the density of a substance to the density of water. It does not have any units because the units of density cancel each other out.
How can you predict if an object will float or sink in a liquid?
-An object will float in a liquid if its density is less than the liquid's density, and it will sink if its density is greater than the liquid's density.
Why does a steel ship float in water despite being made of steel, which has a higher density than water?
-A steel ship floats in water due to its hollow structure, which displaces a volume of water whose weight is greater than the weight of the steel, allowing it to float according to the principle of buoyancy.
What is the purpose of the question posed at the end of the video about the steel ship and water?
-The purpose of the question is to encourage viewers to think critically about the principles of density and buoyancy and to apply them to a real-world scenario, fostering deeper understanding and engagement with the material.
Outlines
🧪 Experimentation with Density and Buoyancy
This paragraph introduces an educational experiment on density, relative density, and flotation. The presenter demonstrates the concept by pouring honey, water, and kerosene into a glass, creating distinct layers due to their different densities. Objects such as a steel nail, a grape, a bottle cap, and a plastic ball are then introduced to the liquids to illustrate their buoyancy. The paragraph concludes with an explanation of density as mass per unit volume, denoted by the symbol rho (ρ), and the formula ρ = M/V, where M is mass and V is volume. The SI and CGS units for density are also discussed, with water's density highlighted as a key reference point.
📚 Understanding Density and Relative Density
The second paragraph delves deeper into the concept of density, explaining that it measures the heaviness or lightness of a material and how tightly packed its particles are. It introduces the relative density, also known as specific gravity, which is the density of a substance compared to water. The units for relative density are discussed, emphasizing that it is unitless as the units cancel out. The relative densities of kerosene and honey are calculated, and the paragraph explains the principles of flotation, stating that an object will float if its density is less than the liquid's density and sink if it is greater. The densities of various objects are estimated based on their behavior in the liquids, and the question of why a steel ship can float despite being made of dense steel is posed to the audience.
🚢 The Paradox of Ship Buoyancy and Learning Opportunities
The final paragraph invites viewers to ponder the paradox of a steel ship floating on water, despite steel being denser than water. It encourages audience interaction by asking them to share their thoughts in the comments. The paragraph then summarizes the key concepts covered in the video: density, relative density, and the conditions for flotation. It also provides a set of formulas for reference and encourages viewers to like, share, and subscribe to the channel for more educational content. The availability of full courses on various subjects is promoted, with an invitation to try them for free, and the paragraph concludes with a reminder to stay connected and continue learning.
Mindmap
Keywords
💡Density
💡Relative Density
💡Flotation
💡Experiment
💡Honey
💡Kerosene
💡Water
💡Steel Nail
💡Grape
💡Bottle Cap
💡Plastic Ball
Highlights
Introduction to an experiment demonstrating density, relative density, and flotation.
Pouring honey, water, and kerosene to create three distinct layers in a glass.
Observing different objects' behavior in various liquids to illustrate the principles of sinking and floating.
Explanation of how liquids arrange themselves based on their densities.
Density defined as mass per unit volume, with the formula ρ = M/V.
Units of density in SI and CGS units, and their conversion.
The density of water is 1 gram per centimeter cube in CGS units.
Relative density is the ratio of a substance's density to water's density, and it is unitless.
Calculating the relative density of kerosene and honey using their densities.
Relative density is also known as specific gravity.
The concept of flotation: objects float if their density is less than the liquid's density.
Determining the density of a steel nail, grape, bottle cap, and plastic ball based on their behavior in liquids.
The paradox of a steel ship floating despite being made of dense material.
Invitation to the audience to comment on the ship's flotation question.
Summary of the key concepts learned in the video: density, relative density, and conditions for flotation.
Promotion of Manocha Academy's courses and resources for further learning.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: