Density
TLDRIn this AP Physics essentials video, Mr. Andersen explores the concept of density, which is a measure of matter's compactness. He demonstrates this by stacking fluids of varying densities and explains that density is calculated as mass divided by volume. The video uses examples like a bowling ball and a volleyball to illustrate density differences and how it affects buoyancy. A PHET simulation is introduced to calculate the density of various materials, showing how objects with different densities float or sink in water. The video concludes with an exercise for students to calculate the density of two objects and predict whether they will float or sink, reinforcing the understanding of density and its practical applications.
Takeaways
- 🧪 Density is a measure of how compact matter is, indicating the mass per unit volume.
- 🌊 Different fluids have different densities, which affects their arrangement when layered.
- 🍯 Maple syrup, being the densest among the examples, is at the bottom, while olive oil, the least dense, is at the top.
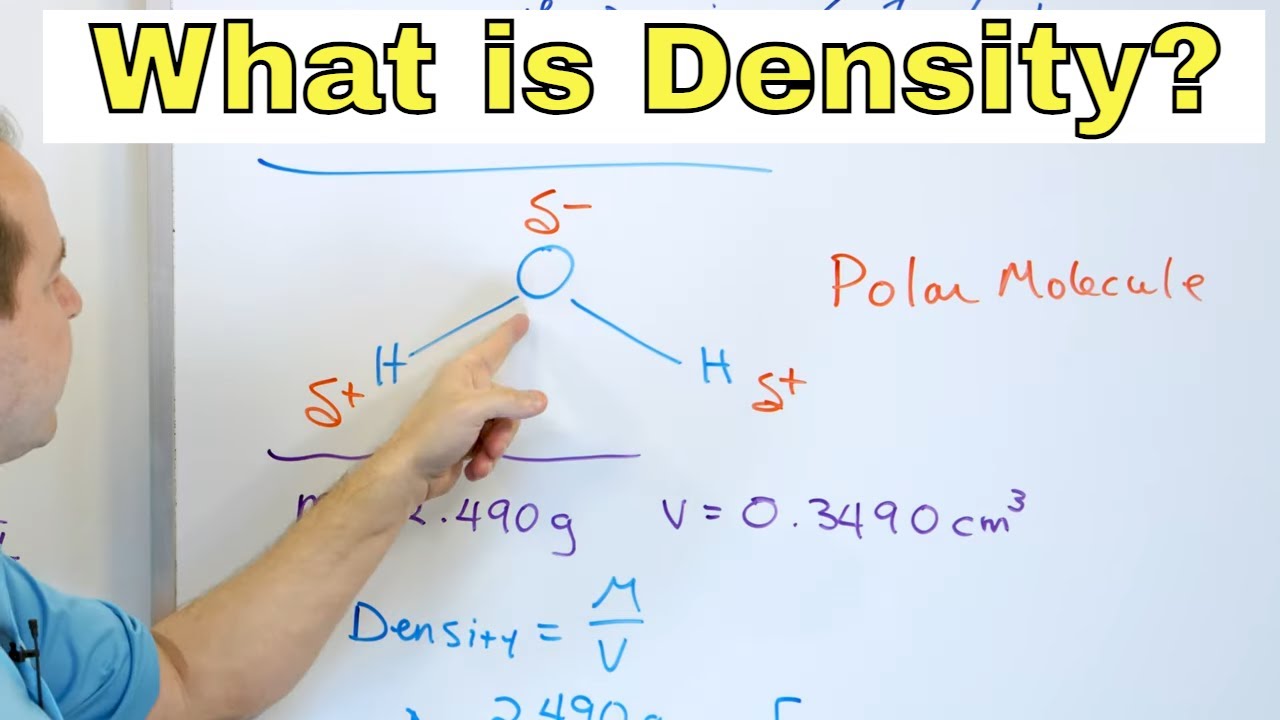
- 🔬 Density is calculated using the formula: Density = Mass / Volume.
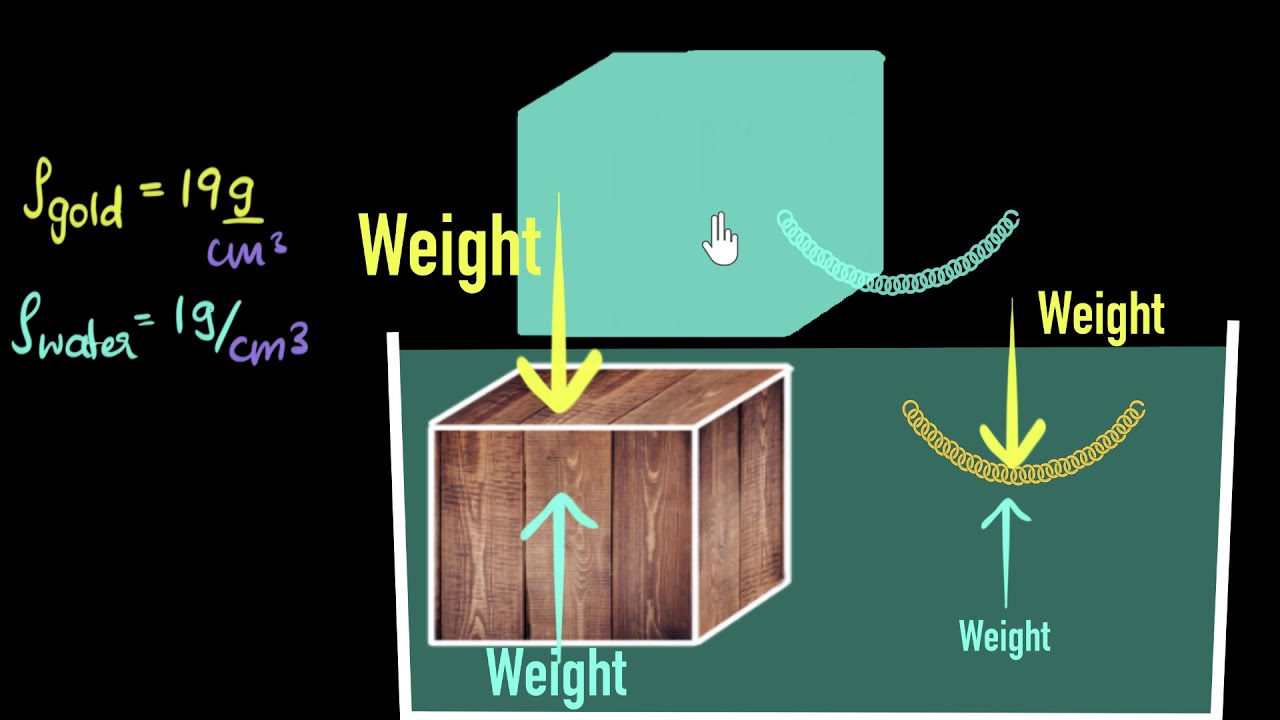
- 📏 Understanding density helps in determining how objects will behave when submerged, such as whether they will float or sink.
- 🏀 Objects with the same mass but different volumes will have different densities.
- 🎾 The volleyball, being mostly filled with air, has a lower density compared to the solid bowling ball.
- 🌡 Changes in temperature can affect the density of materials, causing them to become less or more dense.
- 🧊 Ice floats on water because its density is less than that of water, while a brick, with a higher density, sinks.
- 🛠 The PHET simulation demonstrates how to calculate the density of various materials and predict their buoyancy.
- 📐 To experimentally determine density, one can measure an object's mass and volume or use water displacement to find the volume.
Q & A
What is density?
-Density is a measure of the compactness of matter, which is defined as the mass of an object divided by its volume.
How does density relate to the arrangement of atoms in an object?
-Density is affected by the arrangement of atoms. If objects have the same number of atoms but different volumes, the one with a smaller volume will have a higher density because it is more compact.
Why does maple syrup have a higher density than olive oil?
-Maple syrup has a higher density than olive oil because it is more compact, meaning it has more mass in a given volume compared to olive oil.
What happens to the density of an object when its temperature changes?
-When an object is heated, its molecules move apart, causing an increase in volume and a decrease in density. Conversely, when an object is cooled, its molecules move closer together, decreasing volume and increasing density.
How can you determine if an object will float or sink in water?
-An object will float if its density is less than the density of water. It will sink if its density is greater than that of water.
What is the density of styrofoam in the provided example?
-The density of the styrofoam in the example is 0.15 kilograms per liter.
How does the density of ice compare to that of water?
-The density of ice is 0.92 kilograms per liter, which is less than the density of water (approximately 1 kilogram per liter), allowing it to float.
What is the density of a brick as shown in the PHET simulation?
-The density of the brick in the simulation is 2.0 kilograms per liter, indicating it is much denser than water and will sink.
How can you calculate the density of an object using water displacement?
-You can calculate the density of an object using water displacement by first measuring the object's mass and then the change in water level when the object is submerged. The volume displaced is equal to the change in water level, and density is calculated as mass divided by this volume.
What is the significance of calculating the density of objects in a physics class?
-Calculating the density of objects is significant in a physics class as it helps students understand the concept of density, predict the behavior of objects in different conditions, and apply mathematical skills to real-world scenarios.
Outlines
🧪 Density: The Measure of Compactness in Matter
In this video, Mr. Andersen introduces the concept of density, which is a measure of how compact matter is. He demonstrates this with various fluids of different densities, showing that the most dense substance, like maple syrup, will sink to the bottom while the least dense, like olive oil, will float on top. He explains that density is not solely determined by mass but is calculated by dividing mass by volume. This concept is illustrated with objects of the same mass but different volumes, indicating that density increases with compactness. Mr. Andersen also uses examples like a bowling ball and a volleyball to show how density can be inferred from an object's composition and structure. The video includes a simulation to calculate the density of different materials, such as styrofoam, wood, ice, a brick, and aluminum, each demonstrating how changes in mass and volume affect density and whether the material will float or sink in water.
📚 Calculating and Predicting Object Density
The second part of the video focuses on calculating and predicting the density of objects. Mr. Andersen guides viewers through an experiment where they measure the gravitational mass and volume of objects to determine their density. He uses water displacement as a method to find the volume of objects A and B, which are then used to calculate their densities. The video challenges viewers to predict whether objects will float or sink based on their calculated densities. Object B, with a density less than 1, floats, while object A, with a density greater than 1, sinks. Mr. Andersen emphasizes the importance of being able to predict and experimentally determine the density of objects, either by measuring their volume or using water displacement, as a key skill in understanding physics.
Mindmap
Keywords
💡Density
💡Compactness
💡Mass
💡Volume
💡Matter
💡Bowling Ball
💡Volleyball
💡Heat
💡Cool
💡Water Displacement
💡Float
💡Sink
Highlights
Density is the measure of the compactness of matter.
Different densities of fluids are demonstrated with maple syrup, dish soap, water, wine, vegetable oil, and olive oil.
The most dense material is at the bottom, and the least dense is at the top in a layered setup.
Density is affected by mass and is calculated by dividing mass by volume.
Objects with the same number of atoms can have different densities based on their volume.
A bowling ball has a higher density than a volleyball due to its greater mass and compactness.
Heating a volleyball would decrease its density as molecules move apart.
Cooling a volleyball would increase its density as molecules come closer together.
A PHET simulation is used to explore the densities of different materials.
Styrofoam has a density of 0.15 kg/L, determined by mass and volume.
Wood floats in water due to its density of 0.4 kg/L.
Ice has a density approaching 1.0 kg/L, which is why it floats in water.
A brick has a density of 2.0 kg/L, causing it to sink in water.
Aluminum has a high density, leading to its sinking in water.
Students should be able to calculate the density of an object using its mass and volume.
Water displacement is a method to determine the volume and thus the density of an object.
Object B floats with a density less than 1, while Object A sinks with a density greater than 1.
Students are encouraged to predict and experimentally determine the density of objects.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: