What is Density in Chemistry & Why does Ice Float? - [1-1-12]
TLDRThis lesson delves into the concepts of density and volume in chemistry, emphasizing their importance in understanding how substances behave. The instructor introduces the molecular structure of water, highlighting its bent shape and polarity as key factors influencing its unique property of floating when frozen. The lesson also explores how density is calculated (mass divided by volume) and its implications for whether substances float or sink in liquids. The significance of water's density in supporting life on Earth is underscored, setting the stage for further exploration of molecular properties and their impact on the world around us.
Takeaways
- 📐 The SI unit for length is the meter (m), and volume is measured in cubic meters (m³) or smaller units like cubic centimeters (cm³) and cubic millimeters (mm³).
- 📐 A cubic meter is a volume defined by a box with each side measuring one meter, equivalent to a sugar cube for a cubic centimeter.
- 💧 The density of a substance is calculated as its mass divided by its volume, with units typically expressed in grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL).
- 💧 Water's density at four degrees Celsius is approximately one gram per milliliter, which is a critical value for determining whether objects float or sink in water.
- 💧 Most substances contract when cooled, leading to an increase in density, which causes them to sink in liquids. Ice, however, expands when cooled, resulting in a lower density than liquid water, allowing it to float.
- 🔬 The shape and polarity of water molecules contribute to its unique properties, such as floating on water due to the bent shape and the slight negative charge on the oxygen atom and positive charge on the hydrogen atoms.
- 🔬 The molecular structure of water, with its bent shape and polar nature, leads to the formation of a lattice in ice, which occupies more volume and thus has a lower density compared to liquid water.
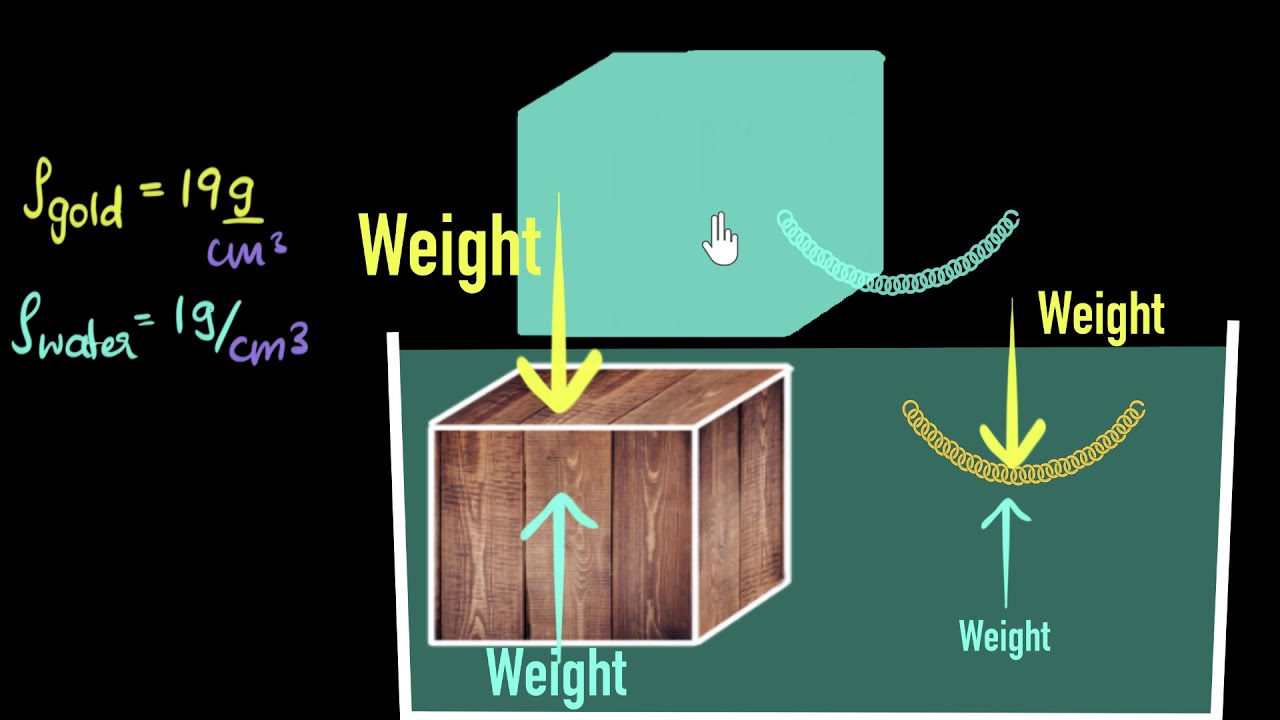
- 📊 To determine if a substance will float on water, compare its density to that of water. If the substance's density is less than one gram per milliliter, it will float; if greater, it will sink.
- 🔢 Density calculations are essential in chemistry for understanding the properties of substances and predicting their behavior in various states and conditions.
- 🔢 Dimensional analysis is a powerful tool in chemistry and other sciences, allowing for the conversion and comparison of units to solve problems involving density and volume.
Q & A
What is the definition of density in the context of chemistry?
-Density is defined as the mass of an object divided by its volume. It is a measure of how much mass is present in a given volume and is typically expressed in grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL).
How does the shape of a water molecule affect its density?
-The water molecule is bent due to the presence of extra electrons around the oxygen atom that push the hydrogen atoms down, creating a V-shape. This bent shape, along with the polar nature of the molecule, leads to the formation of a lattice structure in ice, which increases the volume and thus decreases the density compared to liquid water, allowing ice to float.
What is the significance of water being a polar molecule?
-Being a polar molecule means that there is an uneven distribution of charge within the water molecule, with a slight negative charge on the oxygen atom and slight positive charges on the hydrogen atoms. This polarity is crucial for many of water's unique properties, including its ability to form hydrogen bonds and its lower density in the solid state compared to the liquid state.
Why does the density of most substances increase when they are compressed?
-When a substance is compressed, its volume decreases while its mass remains constant. Since density is calculated as mass divided by volume, a smaller volume results in a larger density value when divided by the same mass.
How does the density of water compare to the density of ice?
-Contrary to most substances, the density of water is less than that of ice. Water has a density of about 0.999 g/cm³ at 4°C, while ice has a lower density, which is why ice floats on water. This is due to the lattice structure formed by water molecules in ice, which occupies more space than the disordered arrangement in liquid water.
What is the SI unit of volume?
-The SI unit of volume is the cubic meter (m³). However, in practical terms, especially in chemistry, smaller units like cubic centimeters (cm³) or milliliters (mL) are commonly used because they are more appropriate for the small quantities typically measured in a laboratory setting.
How is the density of a substance calculated?
-Density (ρ) of a substance is calculated using the formula ρ = m/V, where m is the mass of the substance in grams and V is the volume in cubic centimeters (or milliliters). The resulting density is expressed in grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL).
What is the relationship between the density of an object and its buoyancy in water?
-An object will float on water if its density is less than the density of water. If the object's density is greater than water's density, it will sink. The density of water at 4°C is approximately 1 g/cm³ or 1 g/mL.
Why is the fact that ice floats on water considered 'extremely weird' in the context of the lesson?
-It is considered 'extremely weird' because most substances when solidified (as ice does when becoming water) have a higher density and thus would sink to the bottom in their liquid state. However, ice is an exception with a lower density than liquid water, which allows it to float and has significant implications for life on Earth.
How does the density of a substance change with temperature?
-The density of a substance can change with temperature because heating or cooling can cause expansion or contraction. As a substance gets hotter, it generally gets larger (expands), increasing its volume and decreasing its density. Conversely, cooling a substance usually results in contraction, decreasing its volume and increasing its density.
What is the significance of understanding density in chemistry?
-Understanding density is crucial in chemistry as it helps in predicting the behavior of substances, such as whether they will float or sink in a liquid, how they will react to changes in temperature, and their packing and storage requirements. It's also essential in various chemical calculations and in determining the purity of substances by comparing calculated densities with known densities of pure materials.
Outlines
🔬 Introduction to Density and Volume in Chemistry
The video begins with an introduction to the concepts of density and volume, essential early topics in chemistry. It emphasizes the importance of understanding the units of density and volume, including their significance and how they are measured. The introduction also previews a discussion on the structure of water molecules, highlighting the peculiar bent shape of the water molecule (H2O) and its significance. The bent structure, caused by the distribution of electrons around the oxygen atom, leads to water's unique properties, such as ice floating on liquid water, which has profound implications for life on Earth.
📘 Exploring Units of Volume and Density
This segment delves into the various units of volume, such as cubic meters, cubic centimeters, and milliliters, and their use in different contexts. It explains how these units relate to one another and introduces the concept of derived units in measuring volume and density. The importance of the metric system's base unit of volume, the cubic meter, is discussed alongside more commonly used units in chemistry, such as cubic centimeters and milliliters. The connection between cubic centimeters and milliliters is clarified, establishing their interchangeability and relevance in measuring volume in chemistry.
🧪 Density: Connecting Mass and Volume
The discussion focuses on the definition and calculation of density as the mass of an object divided by its volume. It emphasizes density as a derived unit that combines mass with volume to provide a measure of how much mass is contained within a given volume. This part also explains the use of grams per cubic centimeter or grams per milliliter as practical units for measuring density in chemistry. The concept is further illustrated with examples, including the compression of cotton, to demonstrate how changes in volume affect density.
💧 The Unique Density of Water and Its Implications
This segment explores the unique properties of water, particularly its density and the anomalous behavior of ice. It explains that water reaches its maximum density at 4 degrees Celsius and that ice floats on water because its solid form is less dense than its liquid form. This unusual property is attributed to the molecular structure of water and the arrangement of water molecules in ice, which form a lattice that occupies more volume. This property of water is crucial for life on Earth, as it allows bodies of water to freeze from the top down, insulating the liquid water below and enabling aquatic life to survive in cold climates.
🔍 Detailed Analysis of Water's Molecular Structure
The video goes into depth about the molecular structure of water, emphasizing the polarity of water molecules due to the uneven distribution of electrons. This polarity, with oxygen having a slight negative charge and hydrogens a slight positive charge, leads to the formation of a hydrogen bond network in ice, which is responsible for ice's lower density compared to liquid water. The segment underscores the significance of water's bent molecular shape and its polar nature in determining its unique physical properties, including its ability to float when frozen.
⚖️ Applying Density Concepts to Real-world Problems
The final sections of the video apply the concepts of density and volume to solve real-world chemistry problems, including determining the density of various substances and predicting whether they will float or sink in water. Examples include calculating the density of a penny and a chemical substance, highlighting the practical applications of density in identifying materials and understanding their behavior in different environments. The segment reinforces the importance of density as a key concept in chemistry that connects theoretical knowledge with practical observations.
Mindmap
Keywords
💡Density
💡Volume
💡Buoyancy
💡Water Molecule (H2O)
💡Polar Molecule
💡Molecular Structure
💡Chemical Bonding
💡Electronegativity
💡Cubic Meter and Cubic Centimeter
💡Temperature
Highlights
The lesson introduces the concepts of density and volume in chemistry, emphasizing their importance in understanding various chemical phenomena.
The unit of density is derived from the base units of mass and volume, with the SI unit for density being grams per cubic centimeter (g/cm³) or equivalently grams per milliliter (g/mL).
Density is a critical measure that indicates how much mass is contained within a certain volume, and it can determine whether an object will float or sink in a liquid.
Water's unique molecular structure, being a bent molecule, plays a crucial role in its properties, including its density and the fact that ice floats on water.
The density of water at four degrees Celsius is approximately one gram per milliliter, which is a standard reference point for many chemical calculations.
Most substances contract when cooled, leading to an increase in density, which typically causes them to sink when frozen.
The exception to the general rule of substances sinking when frozen is water, where the solid form (ice) is less dense than the liquid form, allowing it to float.
The讲师强调了水分子的极性特征,这是由于氧原子对电子的强烈吸引导致的分子间形成略微的正负电荷分布。
水分子的弯曲形状和极性特征共同导致了冰的密度低于液态水,这是冰能够在水中漂浮的根本原因。
讲师通过实际问题,如计算便士的密度和讨论四氯化乙烯的浮沉,来实践密度的概念和计算。
讲师解释了如何通过比较物质的密度与水的密度来预测该物质在水中的浮沉行为。
讲师通过例子说明了温度对体积的影响,以及在报告密度时指定温度的重要性。
讲师通过化学问题解答,展示了如何使用密度公式和单位换算来计算未知质量。
讲师强调了在化学计算中使用单位一致性的重要性,并通过实际例子进行了演示。
讲师提到了化学中的一个关键概念——极性分子,水(H2O)就是一个典型的例子。
讲师解释了为什么水的分子结构和极性特征对于地球上的生命至关重要。
讲师通过详细的解释和图示,帮助学生直观理解密度的概念和计算方法。
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: