General Chemistry | Acids & Bases
TLDRThis chemistry tutorial explores the fundamental concepts of acids and bases, introducing the Bronsted-Lowry, Lewis, and Arrhenius theories. It defines strong and weak acids and bases, explains the pH scale, and delves into the significance of the ionization constants Ka and Kb. The video script also covers the auto-ionization of water, represented by the constant Kw, and discusses the practical application of these concepts in buffers, emphasizing the importance of pKa and pkb in maintaining pH stability.
Takeaways
- 🔬 The video discusses the fundamental concepts of acids and bases in chemistry, emphasizing the importance of practice for mastery.
- 📚 Three definitions of acids and bases are covered: Brønsted-Lowry, Lewis, and Arrhenius theories, each offering a unique perspective on proton and electron behavior.
- ⚗️ The script introduces the concept of conjugate acid-base pairs, explaining how an acid and its conjugate base are related through the donation and acceptance of protons.
- 💧 The video defines strong acids and bases, which are substances that completely dissociate in an aqueous solution, and lists examples such as hydrochloric acid and sodium hydroxide.
- 📉 The pH scale is explained as a logarithmic scale measuring the acidity or basicity of a solution, ranging from 0 to 14 with 7 as the neutral point.
- 📈 The relationship between the strength of an acid or base and its conjugate is highlighted, noting that a stronger acid has a weaker conjugate base, and vice versa for bases.
- 🌡 The video introduces the ionization constant (Ka) for weak acids and the base ionization constant (Kb) for weak bases, which quantify the degree of dissociation in solution.
- 🔍 The negative logarithms of Ka and Kb, known as pKa and pKb, are discussed as measures of acid and base strength, with lower values indicating stronger substances.
- 💦 The auto-ionization of water and the ion product of water (Kw) are covered, showing how water can act as both an acid and a base and establishing a constant for water's self-ionization.
- 🔑 The script explains the relationship between Kw, Ka, and Kb, demonstrating how these constants are interrelated and important for understanding acid-base equilibria.
- 🛡️ The concept of buffers is introduced, which are solutions that resist changes in pH, typically composed of a weak acid and its conjugate base, and the importance of pKa in selecting appropriate buffers.
Q & A
What is the main focus of the video?
-The main focus of the video is to discuss acids and bases, including their definitions, properties, and the concepts of strong acids, strong bases, pH scale, and acid-base equilibrium constants.
What are the three different definitions of acids and bases discussed in the video?
-The video discusses Bronsted-Lowry, Lewis, and Arrhenius definitions of acids and bases. Bronsted-Lowry defines acids as proton donors and bases as proton acceptors. Lewis defines acids as electron acceptors and bases as electron donors. Arrhenius defines acids as substances that produce H+ ions in aqueous solutions and bases as those that produce OH- ions.
What is a conjugate acid-base pair?
-A conjugate acid-base pair consists of an acid and its corresponding base that has accepted a proton. For example, when an acid donates a proton (H+), it becomes a conjugate base, and the substance that accepted the proton (like water) becomes the conjugate acid.
How are strong acids defined in the video?
-Strong acids are defined as acids that completely dissociate or deprotonate in an aqueous solution, such as hydrochloric acid, nitric acid, and sulfuric acid.
What is the pH scale and what does it measure?
-The pH scale is a logarithmic scale that ranges from 0 to 14, used to measure the acidity or basicity of a solution. It measures the concentration of H+ ions for acidity and OH- ions for basicity.
What is the relationship between a strong acid and its conjugate base?
-The stronger the acid, the weaker its conjugate base. This is because a strong acid completely dissociates, leaving its conjugate base with less tendency to gain a proton.
What is the significance of the equilibrium constant Ka for weak acids?
-The Ka (acid ionization constant) for weak acids indicates the degree to which the weak acid dissociates in a solution. A larger Ka value means a stronger acid, as it dissociates more in solution.
What is the relationship between Ka and pKa for an acid?
-The pKa is the negative logarithm of the Ka. A lower pKa value indicates a stronger acid because it corresponds to a higher Ka value.
What is the significance of the equilibrium constant Kb for weak bases?
-The Kb (base ionization constant) for weak bases indicates the degree to which the weak base accepts protons or produces hydroxide ions in a solution. A larger Kb value means a stronger base.
What is the relationship between Kw, Ka, and Kb?
-The ion product of water, Kw, is equal to the product of Ka and Kb. This relationship shows that the acidity and basicity of a solution are interrelated through the dissociation of water.
How can you determine the best buffer for a specific pH?
-The best buffer for a specific pH is one where the pKa of the buffer is closest to the desired pH, ideally within plus or minus one unit of the pH.
Outlines
🔬 Introduction to Acids and Bases
This paragraph introduces the topic of acids and bases, emphasizing the importance of practice in mastering chemistry. It outlines the educational goal of NineNine Nerd Science to provide ample practice problems. The paragraph also sets the stage for defining acids and bases according to different theories, discussing strong and weak acids and bases, and explaining concepts like conjugate acid-base pairs, pH scale, and equilibrium constants (Kw, Ka, and Kb).
📚 Definitions of Acids and Bases
The paragraph delves into the definitions of acids and bases from three different perspectives: Brønsted-Lowry, Lewis, and Arrhenius. It explains that a Brønsted-Lowry acid is a proton donor and a base is a proton acceptor. In contrast, a Lewis acid is an electron pair acceptor, and a Lewis base is an electron pair donor. The Arrhenius definition specifies that an acid is a proton donor in an aqueous solution, and a base produces hydroxide ions in the same medium. The paragraph also introduces the concepts of conjugate acids and bases, providing examples to illustrate these definitions.
💪 Strong Acids and Bases
This section focuses on defining strong acids and bases. Strong acids are those that completely dissociate in an aqueous solution, such as hydrochloric acid, nitric acid, and sulfuric acid. The paragraph lists six strong acids and explains that they fully release protons in water. Similarly, strong bases are those that also fully dissociate in water, such as sodium hydroxide and potassium hydroxide. The paragraph establishes the relationship between the strength of an acid and its conjugate base, noting that a stronger acid has a weaker conjugate base.
📉 The pH Scale and Its Significance
The paragraph explains the pH scale as a logarithmic scale ranging from 0 to 14, which measures the acidity or basicity of a solution. It clarifies that a lower pH indicates a higher concentration of H+ ions (more acidic), while a higher pH signifies a higher concentration of OH- ions (more basic). A pH of 7 is neutral, where the concentrations of H+ and OH- ions are equal. The paragraph also discusses the concept of the pH scale as a measure of the concentration of hydronium ions and hydroxide ions, and introduces the relationship pH + pOH = 14.
🌡 Autoionization of Water and KW
This paragraph explores the autoionization of water, where water molecules can act as both an acid and a base, producing H+ and OH- ions. It introduces the constant Kw, which is the equilibrium constant for the autoionization of water, and is equal to 1.0 x 10^-14 at 25 degrees Celsius. The paragraph explains that Kw is the product of the concentrations of H+ and OH- ions, and that these concentrations can be derived from Kw, highlighting the amphoteric nature of water.
🧪 Weak Acids and Bases, Ka, and Kb
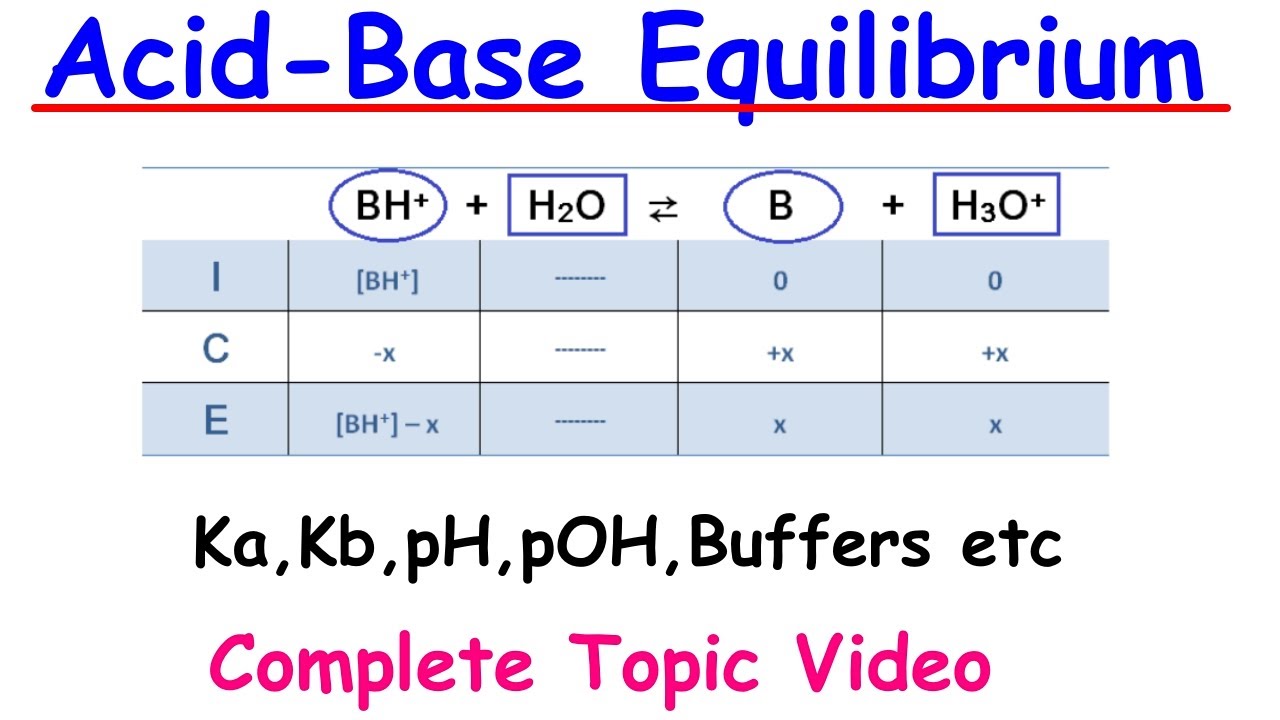
The paragraph discusses weak acids and bases, which do not completely dissociate in solution. It uses acetic acid as an example of a weak acid, explaining that not all acetic acid molecules will donate protons to form H+ and acetate ions. The paragraph introduces the acid ionization constant, Ka, which measures the degree of dissociation of a weak acid. It also explains the relationship between Ka and the strength of an acid, noting that a higher Ka indicates a stronger acid. The paragraph also covers the base ionization constant, Kb, for weak bases like ammonia, and how it measures the degree to which a base accepts protons to form hydroxide ions.
🧪 PKa, PKb, and Buffer Systems
This final paragraph wraps up the discussion by introducing the concepts of PKa and PKb, which are the negative logarithms of Ka and Kb, respectively. It explains the inverse relationship between the strength of an acid or base and its PKa or PKb value. The paragraph also connects these concepts to buffer systems, which are solutions designed to resist pH changes. It emphasizes the importance of selecting a buffer with a PKa close to the desired pH for effective buffering. The paragraph concludes by summarizing the key points covered in the script, setting the stage for future videos on calculating pH values of strong acids and bases.
Mindmap
Keywords
💡Acid
💡Base
💡Conjugate Acid-Base Pair
💡pH Scale
💡Strong Acids
💡Strong Bases
💡Autoionization of Water
💡Ka and Kb
💡pKa and pKb
💡Buffers
Highlights
Introduction to the concept of acids and bases, emphasizing the importance of practice in mastering chemistry.
Explanation of the Bronsted-Lowry definition of acids as proton donors and bases as proton acceptors.
Introduction of the term 'conjugate acid' and 'conjugate base' and their roles in acid-base reactions.
Lewis theory's focus on electron acceptance by acids and electron donation by bases, expanding the definition beyond protons.
A discussion on the Arrhenius definition of acids and bases in the context of aqueous solutions.
Clarification of the relationship between strong acids, strong bases, and their complete dissociation in aqueous solutions.
Listing of the six common strong acids and their dissociation into ions.
Identification of strong bases as those that completely dissociate in water, typically hydroxides of Group 1 and Group 2 metals.
The relationship between the strength of an acid and the weakness of its conjugate base, and vice versa for bases and their conjugate acids.
Introduction of the pH scale as a logarithmic measure of the acidity or basicity of a solution.
Explanation of the pH formula as the negative logarithm of the hydrogen ion concentration.
The significance of the auto-ionization constant (KW) of water and its value at 1.0 x 10^-14.
Derivation of the relationship pH + pOH = 14, connecting the acidity and basicity of a solution.
Brief overview of weak acids and weak bases, their incomplete dissociation, and the role of Ka and Kb constants.
The significance of Ka and Kb as measures of the strength of weak acids and bases, respectively.
Introduction of pKa and pKb as the negative logarithms of Ka and Kb, and their inverse relationship with the strength of acids and bases.
The relationship between KW, Ka, and Kb, and how it applies to both strong and weak acids and bases.
The concept of buffers and their role in maintaining pH stability, with the ideal buffer having a pKa close to the desired pH.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: