Converting Between Moles and Liters of a Gas at STP
TLDRThis educational video script teaches viewers how to convert between moles and liters of a gas at Standard Temperature and Pressure (STP), which is defined as 0°C and 1 atm. It walks through the process using the molar volume of a gas at STP, which is 22.4 liters per mole. The script includes step-by-step problem-solving techniques and conversion factors, and it emphasizes the importance of using these calculations only under STP conditions and for gases, not liquids or solids. It also highlights common mistakes, such as applying STP conversions outside of STP conditions or to non-gaseous substances, and provides additional practice problems to reinforce the concepts.
Takeaways
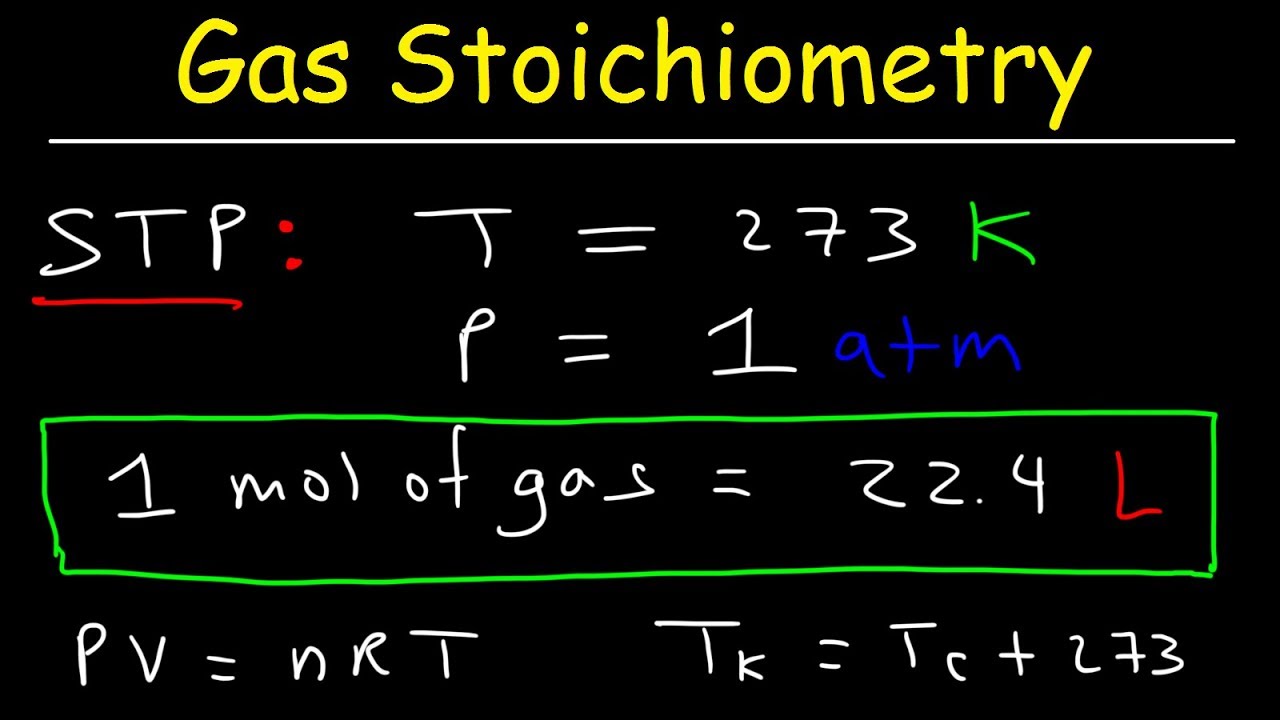
- 🌡️ STP stands for Standard Temperature and Pressure, which is zero degrees Celsius and 1 atm.
- 📏 At STP, one mole of any gas occupies 22.4 liters of volume.
- 🔢 To convert moles to liters at STP, multiply the number of moles by 22.4 liters.
- 🔄 Conversion factors can be used to convert between moles and liters, ensuring units cancel out appropriately.
- 📉 To convert liters to moles at STP, divide the volume in liters by 22.4 liters.
- ⚠️ The relationship of 22.4 liters per mole only applies to gases at STP and not to liquids or solids.
- 🧩 Common mistake: Using the 22.4 liters per mole rule outside of STP conditions (e.g., different temperatures or pressures).
- 💡 Another common mistake: Applying the gas volume relationship to liquids or solids instead of gases.
- 🔍 Always double-check that the substance in question is a gas at STP before applying the 22.4 liters per mole rule.
- 📚 For conditions other than STP, use the ideal gas law (PV=nRT) to solve problems involving gases.
- 📉 Round answers to three significant figures, except when part of a definition or equation where precision is unlimited.
Q & A
What does STP stand for in the context of chemistry?
-STP stands for Standard Temperature and Pressure, which are defined as 0 degrees Celsius and 1 atmosphere of pressure, respectively.
What is the volume occupied by one mole of any gas at STP?
-At STP, one mole of any gas occupies 22.4 liters of volume.
How do you calculate the volume of 3.8 moles of CO2 gas at STP?
-To calculate the volume of 3.8 moles of CO2 gas at STP, multiply the number of moles (3.8) by the volume occupied by one mole of gas at STP (22.4 liters), which gives 3.8 * 22.4 liters.
What is a conversion factor and how is it used in the context of moles and liters?
-A conversion factor is a ratio that shows the equivalence between two quantities. In the context of moles and liters, it is used to convert from moles to liters or vice versa by multiplying the given quantity by the conversion factor that cancels out the unit you want to eliminate.
Why is it incorrect to use the 22.4 liters/mole relationship outside of STP conditions?
-The 22.4 liters/mole relationship is specific to STP conditions (0 degrees Celsius and 1 atm pressure). Using this relationship outside of these conditions, such as at different temperatures or pressures, will yield incorrect results.
What is the common mistake made when calculating the volume of gases at non-STP conditions?
-A common mistake is using the 22.4 liters/mole relationship for gases at non-STP conditions, such as different temperatures or pressures. The correct approach for non-STP conditions is to use the ideal gas law (PV=nRT).
How many moles are in 58.6 liters of nitrogen gas at STP?
-To find the number of moles in 58.6 liters of nitrogen gas at STP, divide the volume (58.6 liters) by the volume occupied by one mole of gas at STP (22.4 liters), which gives 58.6 / 22.4 moles.
Why can't the 22.4 liters/mole relationship be used for liquids or solids?
-The 22.4 liters/mole relationship is only applicable to gases at STP. For liquids or solids, different properties and equations must be used to calculate volume or moles, as the behavior of these states of matter differs significantly from that of gases.
What is the volume occupied by 0.735 moles of O2 gas at STP?
-To find the volume occupied by 0.735 moles of O2 gas at STP, multiply the number of moles (0.735) by the volume occupied by one mole of gas at STP (22.4 liters), which gives 0.735 * 22.4 liters.
How many moles are in 13.0 liters of chlorine gas at STP?
-To determine the number of moles in 13.0 liters of chlorine gas at STP, divide the volume (13.0 liters) by the volume occupied by one mole of gas at STP (22.4 liters), which gives 13.0 / 22.4 moles.
What are the two key considerations when converting between moles and liters of a gas at STP?
-The two key considerations are: 1) Ensure that the conditions specified in the problem are indeed STP (0 degrees Celsius and 1 atm pressure), and 2) Confirm that the substance in question is a gas, as the 22.4 liters/mole relationship does not apply to liquids or solids.
Outlines
🧪 Converting Moles to Liters at STP
This paragraph introduces the concept of converting between moles and liters of a gas at Standard Temperature and Pressure (STP), defined as 0°C and 1 atm. The key information provided is that one mole of any gas occupies 22.4 liters at STP. The paragraph demonstrates how to calculate the volume of 3.8 moles of CO2 gas at STP, using both direct multiplication and conversion factors. It emphasizes the importance of using the correct conversion factor to cancel out the units and arrive at the volume in liters. The final answer is given as 85.1 liters, rounded to three significant figures.
📚 Calculating Moles from Liters and Common Mistakes
This section continues the theme of gas calculations at STP, focusing on determining the number of moles from a given volume. It illustrates the process using 58.6 liters of nitrogen gas, showing both the division method and the use of conversion factors to find the moles of gas. The result is 2.62 moles, rounded to three significant figures. The paragraph then discusses common mistakes made when calculating volumes and moles outside of STP conditions or when dealing with substances that are not gases, such as liquids or solids. It stresses the importance of adhering to STP conditions and the state of matter (gas) when using the 22.4 liters per mole relationship.
🔍 Further Practice with Moles and Liters Conversions
The final paragraph provides additional examples to practice converting between moles and liters of gas at STP. It includes calculating the volume occupied by 0.735 moles of O2 gas and determining the number of moles in 13.0 liters of chlorine gas at STP. The calculations are performed using the established relationship between moles and volume at STP, with conversion factors to simplify the process. The outcomes are 16.5 liters for the volume of O2 gas and 0.580 moles for the amount of chlorine gas, reinforcing the principles introduced earlier in the script. The paragraph also reiterates the importance of ensuring that the conditions are at STP and that the substance is a gas to apply the 22.4 liters per mole rule correctly.
Mindmap
Keywords
💡Moles
💡Liters
💡STP (Standard Temperature and Pressure)
💡Conversion Factors
💡Molar Volume
💡Ideal Gas Law
💡Significant Figures
💡Equivalence
💡Common Mistakes
💡Gas
Highlights
The video teaches how to convert between moles and liters of gas at STP (Standard Temperature and Pressure).
STP is defined as zero degrees Celsius and 1 atm.
At STP, one mole of any gas occupies 22.4 liters.
Demonstration of converting 3.8 moles of CO2 gas to liters at STP.
Use of conversion factors to solve problems involving moles and liters.
The method to calculate the volume of 3.8 moles of CO2 gas at STP is shown.
Final answer for the volume of 3.8 moles of CO2 gas is 85.1 liters.
Explanation of how to find moles from liters of gas at STP.
Calculation of moles in 58.6 liters of nitrogen gas at STP.
Common mistakes when not adhering to STP conditions (e.g., different temperatures and pressures).
Clarification that the 22.4 liters per mole rule only applies to gases at STP.
Highlighting the incorrect application of the 22.4 liters per mole rule to non-gaseous states.
Two additional problems are solved to practice mole and liter conversions at STP.
Conversion of 0.735 moles of O2 gas to liters at STP.
Conversion of 13.0 liters of chlorine gas to moles at STP.
Emphasis on ensuring the problem specifies STP conditions and involves a gas.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: