Conservation of Charge in Reactions
TLDRThis AP Physics essentials video 90, presented by Mr. Andersen, delves into the conservation of charge in nuclear reactions, using uranium 235 fission as an example. It explains how the atomic number (proton count) and mass number (sum of protons and neutrons) are conserved during nuclear reactions, even when elementary particles are created or destroyed. The video covers alpha decay, where uranium 238 transforms into thorium 234 by emitting an alpha particle (2 protons and 2 neutrons), and discusses beta decay, highlighting both beta minus decay (e.g., carbon 14 to nitrogen 14) and beta plus decay (e.g., magnesium 23 to sodium 23). The video emphasizes the importance of charge conservation in nuclear processes, providing a clear understanding of the fundamental principles governing nuclear reactions.
Takeaways
- 🔬 The script discusses the conservation of charge in nuclear reactions, specifically using the fission of uranium 235 as an example.
- 📚 In the fission of uranium 235, it breaks apart into barium 141 and krypton 92, with the conservation of charge maintained before and after the reaction.
- ⚛️ The script emphasizes that in nuclear reactions, the charge of electrons can be ignored, focusing instead on the positive charge within the nucleus and the mass number.
- 🧬 The atomic number, which is the number of protons, determines the identity of an atom, as illustrated with uranium having 92 protons.
- 📉 The conservation of mass is also highlighted, with the mass number on both sides of the nuclear reaction equation being equal.
- 🚫 The script clarifies that in nuclear reactions, the creation or destruction of elementary particles still adheres to the conservation of charge.
- 🌐 The video explains that in chemical reactions, electrons are crucial, whereas in nuclear reactions, the focus is on the nucleus and its constituents, protons and neutrons.
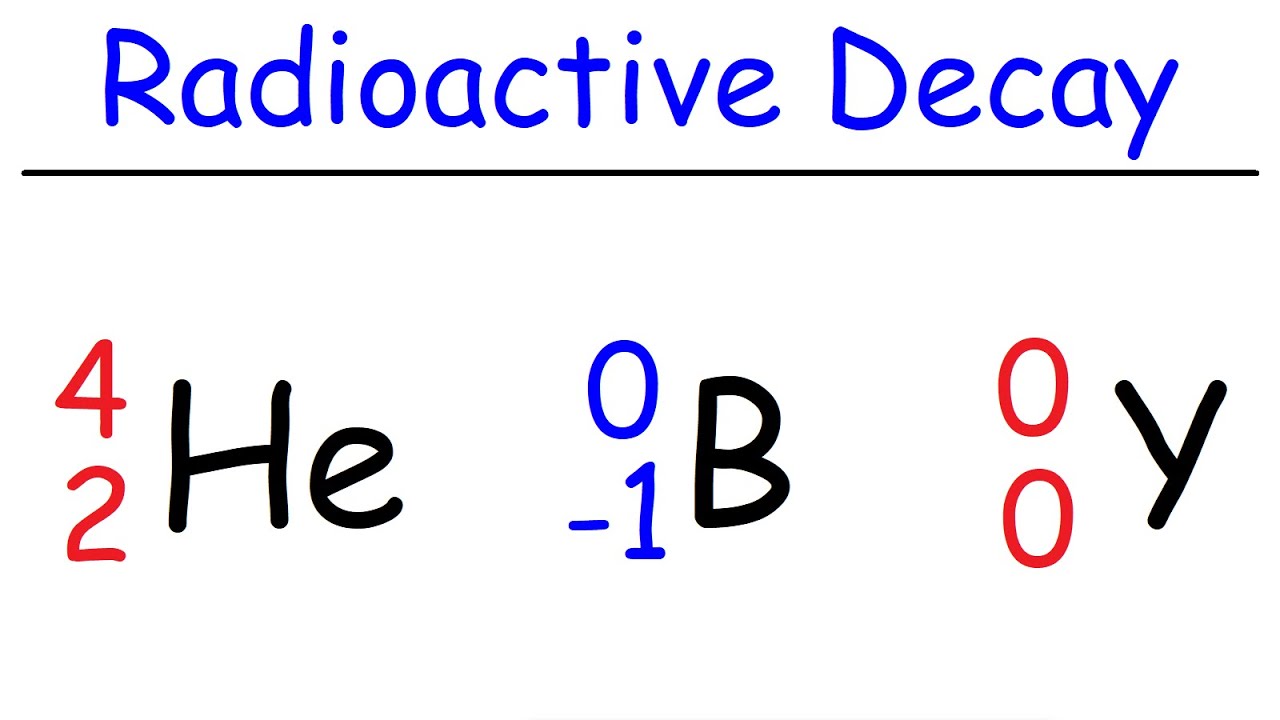
- 💥 Alpha decay is described as the emission of an alpha particle, which contains 2 protons and 2 neutrons, resulting in a 2+ charge.
- 🔄 The script provides an example of alpha decay with uranium 238 turning into thorium 234, illustrating the conservation of both mass and charge.
- 🚀 Beta minus decay is explained as the transformation of a neutron into a proton within the nucleus, accompanied by the emission of an electron and an electron antineutrino.
- 🛑 Beta plus decay involves the conversion of a proton into a neutron, resulting in the emission of a positron and an electron neutrino, while conserving charge.
Q & A
What is the main topic of the video?
-The main topic of the video is the conservation of charge in nuclear reactions, specifically focusing on fission of uranium 235.
Why can electrons be ignored in nuclear reactions?
-Electrons can be ignored in nuclear reactions because the focus is on the nucleus and the nucleons (protons and neutrons), and electrons do not play a significant role in the conservation of charge in these reactions.
What happens when uranium 235 absorbs a neutron?
-When uranium 235 absorbs a neutron, it undergoes fission and quickly breaks apart into barium 141 and krypton 92, along with the release of additional neutrons.
What is the charge of a neutron?
-A neutron has a charge of 0, which means it is electrically neutral.
What is the atomic number of uranium?
-The atomic number of uranium is 92, which represents the number of protons in the nucleus of a uranium atom.
How does the conservation of charge apply to nuclear reactions?
-The conservation of charge in nuclear reactions means that the total charge before the reaction is equal to the total charge after the reaction, even if elementary particles are created or destroyed.
What is alpha decay?
-Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which consists of 2 protons and 2 neutrons, resulting in a 2+ charge.
What is produced during beta minus decay?
-During beta minus decay, a neutron in the nucleus is converted into a proton, an electron (beta particle) is emitted, and an electron antineutrino is also produced.
What is the difference between beta minus and beta plus decay?
-In beta minus decay, a neutron is converted into a proton and an electron is emitted. In contrast, beta plus decay involves the conversion of a proton into a neutron with the emission of a positron (positive electron) and an electron neutrino.
What is the role of the electron antineutrino in beta minus decay?
-The electron antineutrino is produced alongside the electron during beta minus decay. It does not have any charge or mass but is part of the conservation of lepton number in the reaction.
How does the conservation of mass apply to nuclear reactions?
-The conservation of mass in nuclear reactions states that the total mass number before the reaction is equal to the total mass number after the reaction, taking into account the mass of emitted particles.
Outlines
🔬 Conservation of Charge in Nuclear Reactions
This paragraph introduces the concept of conservation of charge in nuclear reactions, specifically using the example of uranium 235 fission. It explains that the positive charge (number of protons) and mass number must remain constant before and after the reaction. The fission of uranium 235 into barium 141 and krypton 92, along with three neutrons, is detailed to illustrate this principle. The paragraph emphasizes that while electrons are crucial in chemical reactions, they can be ignored in nuclear reactions, focusing instead on the nucleus and its constituents, protons, and neutrons. The importance of balancing the charges and mass numbers during nuclear reactions is highlighted.
🌀 Types of Nuclear Decay and Charge Conservation
This paragraph delves into different types of nuclear decay, emphasizing the conservation of charge throughout these processes. It starts with alpha decay, where uranium 238 decays into thorium 234 by emitting an alpha particle, which consists of 2 protons and 2 neutrons, resulting in a 2+ charge. The paragraph then discusses beta minus decay, exemplified by the decay of carbon 14 into nitrogen 14, where a neutron is converted into a proton, and an electron (beta particle) is emitted to maintain charge balance. Additionally, beta plus decay is introduced, where magnesium 23 decays into sodium 23 with the emission of a positron and an electron neutrino. The summary underscores the importance of understanding how charge is conserved in nuclear reactions, even when elementary particles are created or destroyed.
Mindmap
Keywords
💡Conservation of Charge
💡Nuclear Reactions
💡Fission
💡Uranium-235
💡Neutron
💡Mass Number
💡Alpha Decay
💡Beta Minus Decay
💡Beta Plus Decay
💡Electron
💡Neutrino
Highlights
The video discusses the conservation of charge in nuclear reactions, specifically in the fission of uranium 235.
Uranium 235 breaks apart into barium 141 and krypton 92 when hit with a neutron, conserving charge before and after the reaction.
Electrons can be ignored in nuclear reactions, focusing on the positive charge inside the nucleus and mass number.
The equation for uranium fission includes a neutron, uranium atom, barium, krypton, and three neutrons, conserving both charge and mass number.
A neutron has a charge of 0 and a mass number of 1, while uranium has a charge of 92 and a mass number of 235.
In nuclear reactions, charge conservation holds even when elementary particles are created or destroyed.
Nuclear decay is a type of nuclear reaction where charge conservation is maintained.
Chemical reactions differ from nuclear reactions, with the former focusing on electron transfer.
In nuclear reactions, only the nucleus is considered, ignoring electrons unless they are created in the process.
Alpha decay involves the emission of an alpha particle, which consists of 2 protons and 2 neutrons, carrying a 2+ charge.
Uranium 238 undergoing alpha decay produces thorium 234, conserving both mass and charge.
Beta minus decay is exemplified by the decay of carbon 14 into nitrogen 14, involving the conversion of a neutron into a proton and the emission of an electron.
Beta plus decay, such as the decay of magnesium 23 into sodium 23, produces a positron and an electron neutrino.
In beta decay, charge conservation is maintained by balancing the creation of positive and negative charges.
The video concludes with the importance of understanding charge conservation in analyzing nuclear reactions.
Transcripts
Browse More Related Video

Conservation of Nucleon Number

How To Balance Nuclear Equations In Chemistry
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry

Alpha Particles, Beta Particles, Gamma Rays, Positrons, Electrons, Protons, and Neutrons

Nuclear Physics: Crash Course Physics #45

Alpha Decay
5.0 / 5 (0 votes)
Thanks for rating: