Alpha Particles, Beta Particles, Gamma Rays, Positrons, Electrons, Protons, and Neutrons
TLDRThis video delves into the fascinating world of radioactive decay within nuclear chemistry. It explains the characteristics and symbols of key particles involved in decay processes, such as the alpha particle, beta particle, positron, proton, neutron, and gamma particle. The script then illustrates how these particles interact during different decay types, including beta decay, positron production, electron capture, and alpha particle production. Through examples like the transformation of nitrogen-13 to oxygen-13 and the capture of an electron by arsenic-73 resulting in germanium, the video clarifies the conservation of mass and the changes in atomic and neutron numbers. The summary emphasizes the importance of balancing mass and charge in nuclear reactions and provides a foundational understanding of radioactive decay mechanisms.
Takeaways
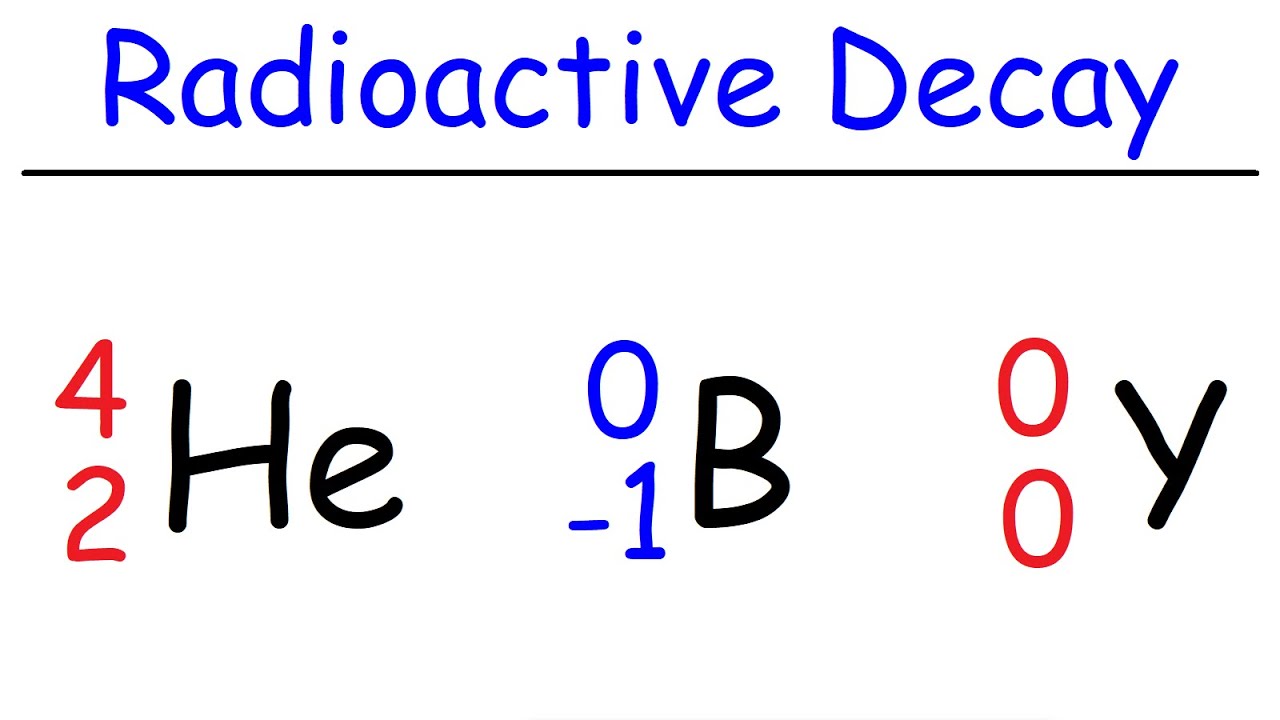
- 🧬 Alpha particles are equivalent to the nucleus of a helium atom, with a mass of 4 and a charge of +2.
- 🔋 Beta particles are essentially electrons, with a mass of 0 and a charge of -1.
- 🎭 The positron is the antiparticle of an electron, having a mass of 0 and a charge of +1.
- ⚛️ Protons are much more massive than electrons, with a mass of 1 and a charge of +1.
- ⚙️ Neutrons are neutral, with a mass of 1 and no charge.
- 🌟 Gamma particles are high-energy photons, with a mass of 0 and no charge.
- ➡️ In beta decay, the atomic number increases while the mass remains constant, resulting from a neutron converting to a proton and an electron.
- 🔁 Positron production decreases the atomic number, as a proton converts into a neutron and emits a positron.
- ⚡️ When a positron meets an electron, they annihilate each other, producing gamma radiation.
- 🕸️ Electron capture occurs when a nucleus absorbs an electron, converting a proton into a neutron and increasing the neutron count.
- 📉 Alpha particle production results in the element losing two in atomic number and four in mass number, as the nucleus emits an alpha particle.
Q & A
What is an alpha particle and what is it equivalent to?
-An alpha particle has a mass of four and a charge of two, and it is equivalent to the nucleus of a helium atom.
What are the characteristics of a beta particle?
-A beta particle has a mass of zero and a charge of negative one, and it is essentially the same as an electron.
How is a positron different from an electron?
-A positron is the antiparticle of an electron, having the same mass (zero) but with a positive charge of one instead of a negative charge.
What is the mass and charge of a proton?
-A proton has a mass of one and a charge of one, making it much more massive than an electron.
What are the properties of a neutron?
-A neutron is neutral, with a mass of one and a charge of zero.
How is a gamma particle described?
-A gamma particle is a high-energy photon, with a mass of zero and no charge.
What happens to the atomic number during beta decay?
-During beta decay, the atomic number increases because a neutron in the nucleus is converted into a proton and an electron, with the electron escaping the nucleus.
What is the effect of positron production on the atomic number and neutron count?
-Positron production decreases the atomic number as a proton is converted into a neutron and a positron, and it increases the neutron count by one.
What occurs during electron capture?
-During electron capture, the nucleus of an atom absorbs one of its inner core electrons, leading to a decrease in the atomic number by one and an increase in the neutron count by one.
How does alpha particle production affect the atomic number and mass number of an element?
-Alpha particle production decreases both the atomic number and the mass number by two and four, respectively, as the alpha particle consists of two protons and two neutrons.
What is the outcome when a positron meets an electron?
-When a positron meets an electron, they annihilate each other, and the result is the formation of a gamma particle.
What is the key to balancing nuclear reactions?
-The key to balancing nuclear reactions is ensuring that the masses and atomic numbers, or charges, are the same on both sides of the equation.
Outlines
🔬 Radioactive Decay Basics and Particles
This paragraph introduces the concept of radioactive decay within nuclear chemistry. It discusses various particles including alpha particles (with a mass of four and a charge of two, equivalent to a helium nucleus), beta particles (identical to electrons with a mass of zero and a charge of negative one), positrons (antiparticles of electrons, with a positive charge), protons (mass of one and a charge of one), neutrons (neutral with a mass of one), and gamma particles (high-energy photons with zero mass and charge). The paragraph also explains how to determine the resulting element after beta decay using mass and charge conservation, using nitrogen-13 as an example that decays into oxygen-13.
🚀 Beta Decay and Positron Production
The second paragraph delves into the effects of beta decay, which increases the atomic number without changing the mass, as seen in the transformation of nitrogen into oxygen. It also covers positron production, where the atomic number decreases, as exemplified by the conversion of nitrogen into carbon. The paragraph explains that during positron production, a proton is converted into a neutron and a positron, and touches on the annihilation of a positron and an electron to form a gamma particle. Lastly, it introduces electron capture, where an atom's nucleus absorbs an inner core electron, leading to the formation of a new element with a decreased atomic number, using arsenic transforming into germanium as an example.
⚛️ Electron Capture and Alpha Particle Production
The final paragraph discusses electron capture in more detail, where a proton and an electron combine to form a neutron, resulting in a decrease in the atomic number and an increase in the neutron count, as shown with arsenic turning into germanium. The paragraph concludes with an introduction to alpha particle production, where an element such as polonium (with an atomic number of 84) emits an alpha particle, resulting in the formation of lead (with an atomic number of 82). It emphasizes the importance of balancing the equation in terms of mass and charge to ensure a correct understanding of the nuclear reaction.
Mindmap
Keywords
💡Radioactive Decay
💡Alpha Particle
💡Beta Particle
💡Positron
💡Proton
💡Neutron
💡Gamma Particle
💡Electron Capture
💡Atomic Number
💡Mass Number
💡Nuclear Chemistry
Highlights
Alpha particles have a mass of four and a charge of two, equivalent to the nucleus of a helium atom.
Beta particles have a mass of zero and a charge of negative one, essentially the same as an electron.
Positrons are the antiparticles of electrons with a positive charge and a mass of zero.
Protons are significantly more massive than electrons, with a mass of one and a charge of one.
Neutrons are neutral, with a mass of one and a charge of zero.
Gamma particles are high-energy photons with zero mass and zero charge.
In beta decay, the atomic number increases, while the mass remains constant.
Beta decay involves the conversion of a neutron into a proton and an electron, with the electron being emitted.
Positron production decreases the atomic number, as a proton converts into a neutron and a positron.
When a positron meets an electron, they annihilate each other, forming a gamma particle.
Electron capture occurs when an atom's nucleus captures one of its inner core electrons, resulting in a change of the element.
During electron capture, a proton combines with an electron to form a neutron, increasing the neutron number by one.
Alpha particle production involves the emission of an alpha particle, resulting in a decrease in the atomic number by two and the mass number by four.
The element transformed by alpha particle production can be determined by balancing the mass and atomic numbers.
Radioactive decay processes include alpha decay, beta decay, positron production, electron capture, and their effects on atomic structure.
The periodic table is used to identify elements based on atomic numbers in radioactive decay processes.
The neutron number can be calculated as the difference between the mass number and the atomic number.
Radioactive decay is a fundamental concept in nuclear chemistry, with practical applications in various fields.
The video provides an introduction to different types of radioactive decay, explaining their effects on atomic structure.
Transcripts
Browse More Related Video
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry

Conservation of Charge in Reactions

15.2 Routes of Nuclear Decay, Fission, and Fusion | High School Chemistry

10. Radioactive Decay Continued

8. Radioactive Decay — Modes, Energetics, and Trends

How To Balance Nuclear Equations In Chemistry
5.0 / 5 (0 votes)
Thanks for rating: