Conservation of Nucleon Number
TLDRIn this AP Physics essentials video, Mr. Andersen explains the principle of conservation of nucleon number and charge in nuclear reactions and radioactive decay. He introduces nucleons as protons and neutrons in an atom's nucleus and discusses how they behave in processes like fission, fusion, and decay. The video covers AZX notation to represent particles and illustrates how nucleon number and charge are conserved in reactions involving uranium, deuterium, tritium, and in decay processes like alpha and beta decay. The explanation includes the transformation of elements and the release of energy, emphasizing the fundamental laws governing nuclear physics.
Takeaways
- 🚀 Nucleons, which include protons and neutrons, are the fundamental particles found inside the nucleus of an atom.
- 🌌 Neutron stars are incredibly dense celestial bodies formed after a supernova, where most protons are converted into neutrons.
- 🔬 The conservation of nucleon number and charge is a fundamental principle in nuclear reactions and radioactive decay, ensuring the balance of protons, neutrons, and their charges before and after the process.
- ⚛️ Nuclear reactions can be categorized into fission, where a nucleus is split, and fusion, where two nuclei combine to form a new one.
- 📊 AZX notation is used to denote the atomic number (Z), mass number (A), and chemical symbol (X) of particles, providing a shorthand for the number of protons and neutrons.
- 💥 In fission, a heavy nucleus like uranium-235 can be split by a neutron into lighter elements, such as krypton-92 and barium-141, along with additional neutrons.
- 🔥 Fusion, as occurs in the sun with isotopes of hydrogen like deuterium and tritium, results in the formation of helium and a release of a large amount of energy.
- 💫 Radioactive decay involves the emission of radiation in various forms, including alpha, beta, and gamma radiation, each with different penetrating powers and energy levels.
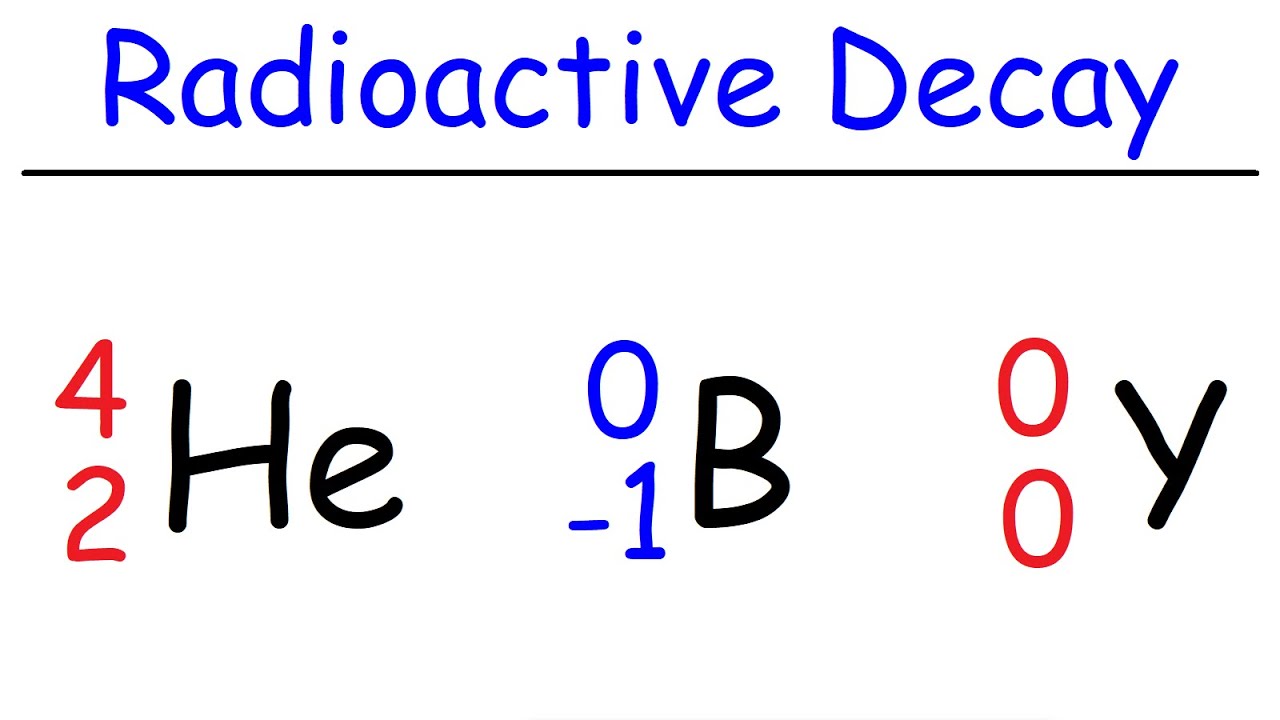
- 📉 Alpha decay involves the release of an alpha particle, which consists of two protons and two neutrons, resulting in a decrease in the mass number and a change in the atomic number of the decaying atom.
- 🔄 Beta decay is a process where a neutron is converted into a proton, emitting an electron and an electron antineutrino, thus changing the atomic number of the atom.
- 🌟 Gamma decay involves the emission of high-energy electromagnetic radiation without a change in the atomic or mass number, often indicating an excited state of the atom.
Q & A
What are nucleons and why are they important in nuclear reactions?
-Nucleons are protons and neutrons found within the nucleus of an atom. They are important in nuclear reactions because the conservation of nucleon number and charge must always be maintained in every nuclear reaction and radioactive decay.
What happens during a supernova that leads to the formation of a neutron star?
-During a supernova, a massive star explodes, and due to the immense gravity, many of the protons are converted into neutrons, resulting in a neutron star which is incredibly dense and has about twice the mass of our sun but with a diameter of only around 7 miles.
What are the two main types of nuclear reactions discussed in the script?
-The two main types of nuclear reactions discussed are fission, where a nucleus is split into smaller parts, and fusion, where two nuclei combine to form a new nucleus.
How is the conservation of nucleon number demonstrated in the fission of uranium-235?
-In the fission of uranium-235, the conservation of nucleon number is demonstrated by the fact that the total number of nucleons (protons and neutrons) before the reaction (uranium-235 plus one neutron) is equal to the total number after the reaction (krypton-92, barium-141, and three neutrons).
What is the AZX notation and how does it help in understanding nuclear reactions?
-The AZX notation is a system for writing down the number of protons and indirectly the number of neutrons inside any particle. 'A' represents the mass number (total number of protons and neutrons), 'Z' is the atomic number (number of protons), and 'X' is the chemical symbol. This notation helps in understanding nuclear reactions by providing a clear way to track the conservation of nucleon number and charge.
How does the fusion process in the sun involving deuterium and tritium result in the formation of helium?
-In the sun, deuterium (hydrogen with one proton and one neutron) and tritium (hydrogen with one proton and two neutrons) fuse together to form helium, which has two protons and two neutrons. This process also releases a neutron and a large amount of energy.
What is alpha decay and what particles are emitted during this process?
-Alpha decay is a type of radioactive decay where an alpha particle, which consists of two protons and two neutrons, is emitted from a nucleus. This results in the nucleus losing two protons and two neutrons, thus changing its atomic number and mass number.
What occurs during beta decay and how does it affect the atomic number of an atom?
-During beta decay, a neutron within the nucleus is converted into a proton, an electron (beta particle) is emitted, and an electron antineutrino is produced. This process increases the atomic number of the atom by one, as it effectively adds a proton to the nucleus.
What is the role of gamma radiation in radioactive decay and how is it different from alpha and beta decay?
-Gamma radiation is a high-energy electromagnetic ray that can be emitted during radioactive decay. Unlike alpha and beta decay, which involve the emission of particles from the nucleus and change the atomic number or mass number, gamma radiation does not alter the composition of the nucleus but rather involves the energy of the electrons in the excited state.
How can you determine the product of a radioactive decay using the conservation laws?
-By applying the conservation of nucleon number and electric charge, you can determine the product of a radioactive decay. For example, if the mass number is conserved and the atomic number changes, you can deduce the new element formed and its properties.
Outlines
🔬 Conservation of Nucleon Number in Nuclear Reactions
This paragraph introduces the concept of nucleon number conservation in nuclear reactions and radioactive decay. Nucleons, which include protons and neutrons, are fundamental to the nucleus of an atom. The video uses the example of a neutron star to illustrate the extreme conditions that can alter the balance of protons and neutrons. The principle of conservation dictates that the number of nucleons and their total charge must remain constant before and after any nuclear reaction or decay. Two types of nuclear reactions are discussed: fission, where a nucleus is split, and fusion, where two nuclei combine. The AZX notation is introduced as a method to denote the number of protons and neutrons in a particle, exemplified with uranium-235. The paragraph emphasizes the importance of understanding nucleon conservation in physics, distinct from the conservation of atoms in chemistry.
📚 Radioactive Decay and Conservation Laws
The second paragraph delves into the specifics of radioactive decay, which is the process by which an atom emits radiation and energy. Three primary types of radiation are covered: alpha, beta, and gamma, each with different penetration capabilities and energy levels. Alpha decay involves the emission of an alpha particle, consisting of two protons and two neutrons, leading to a decrease in the atomic number and mass number of the decaying nucleus. Beta decay is highlighted as a process where a neutron is converted into a proton, emitting an electron and an electron antineutrino, thus increasing the atomic number while keeping the mass number constant. Gamma decay is described as the emission of high-energy electromagnetic radiation from an excited nucleus, which does not alter the nucleon number or charge but indicates excess energy. The paragraph concludes with an emphasis on applying the conservation of nucleon number and electric charge to understand and predict the outcomes of radioactive decays and nuclear reactions.
Mindmap
Keywords
💡Nucleon
💡Neutron Star
💡Fission
💡Fusion
💡Nuclear Decay
💡A Z X Notation
💡Alpha Particle
💡Beta Particle
💡Gamma Radiation
💡Conservation Laws
Highlights
Nucleons are protons and neutrons found within an atom's nucleus.
Neutron stars are incredibly dense objects formed after a supernova, with many protons converted into neutrons.
Conservation of nucleon number and charge is a fundamental principle in nuclear reactions and radioactive decay.
Nuclear reactions can be categorized into fission, where a nucleus is split, and fusion, where two nuclei combine.
AZX notation is a method to denote the number of protons and neutrons in a particle.
Uranium-235 has 92 protons and 143 neutrons, as determined by its mass number.
In physics, the focus is on nucleons rather than electrons, unlike in chemistry.
Fission of uranium-235 with a neutron results in krypton-92, barium-141, and three additional neutrons.
Conservation laws are applied by writing nuclear reactions in AZX notation to ensure nucleon number and charge are balanced.
Fusion in the sun involves isotopes of hydrogen, deuterium and tritium, fusing to form helium and release energy.
Alpha decay involves the release of an alpha particle, which contains two protons and two neutrons.
Uranium-238 decays into thorium through alpha decay, conserving nucleon number and charge.
Beta decay is the conversion of a neutron into a proton, with the emission of an electron and an electron antineutrino.
Carbon-14 undergoes beta decay to become nitrogen, with conservation of nucleon number and electric charge.
Gamma decay involves the emission of high-energy electromagnetic radiation without change in nucleon number or charge.
Excited states of atoms, such as in gamma decay, indicate energy within the electron positions.
The video teaches the application of conservation laws to radioactive decay and nuclear reactions.
Transcripts
Browse More Related Video

Conservation of Charge in Reactions

Alpha Particles, Beta Particles, Gamma Rays, Positrons, Electrons, Protons, and Neutrons

Half-Life and Radioactive Decay

How To Balance Nuclear Equations In Chemistry
Alpha Decay, Beta Decay, Gamma Decay - Electron Capture, Positron Production - Nuclear Chemistry

Nuclear Physics: Crash Course Physics #45
5.0 / 5 (0 votes)
Thanks for rating: