Nuclear Physics: Crash Course Physics #45
TLDRAlbert Einstein's famous equation E=mc^2 shows that mass and energy are equivalent. This concept is key to nuclear physics, which studies atomic nuclei and the conversion of mass into energy. Nuclei consist of protons and neutrons held together by the strong nuclear force. Unstable nuclei can decay into more stable states, releasing energetic particles. Major types of radioactive decay include alpha decay, where the nucleus emits helium nuclei; beta decay, where neutrons convert into protons by emitting electrons; and gamma decay, which releases high-energy photons. These decays allow the release of nuclear binding energy, which can be harnessed to generate power.
Takeaways
- 📡 E=mc^2 represents the equivalence of mass and energy, foundational to nuclear physics.
- 🔮 Atomic nuclei are composed of protons and neutrons, known collectively as nucleons.
- 💧 Atomic number indicates the number of protons in a nucleus, defining the element.
- 🌠 Mass number is the total count of protons and neutrons, determining isotopes.
- 💎 Isotopes have the same atomic number but different mass numbers.
- 🖥 Unified atomic mass units measure nuclear masses, essential for understanding nuclear reactions.
- 💥 Binding energy is the energy needed to disassemble a nucleus into its components.
- 🛠 The strong nuclear force holds protons and neutrons together, overcoming electromagnetic repulsion.
- 💲 Radioactivity involves the decay of unstable nuclei into more stable forms.
- 💾 Alpha, beta, and gamma decays are types of nuclear reactions, each with unique characteristics.
Q & A
What does the equation E=mc^2 mean?
-E=mc^2 is Einstein's famous equation showing that energy (E) and mass (m) are equivalent and can be converted into one another. The speed of light squared (c^2) is the conversion factor between mass and energy.
What are isotopes?
-Isotopes are variants of a chemical element that have different numbers of neutrons. Isotopes of an element have the same atomic number (number of protons) but different mass numbers (total protons + neutrons).
What is binding energy?
-Binding energy is the energy required to break a nucleus into its component protons and neutrons. Stable nuclei have less mass than their constituent parts, with the missing mass accounted for by the binding energy holding the nucleus together.
What is alpha decay?
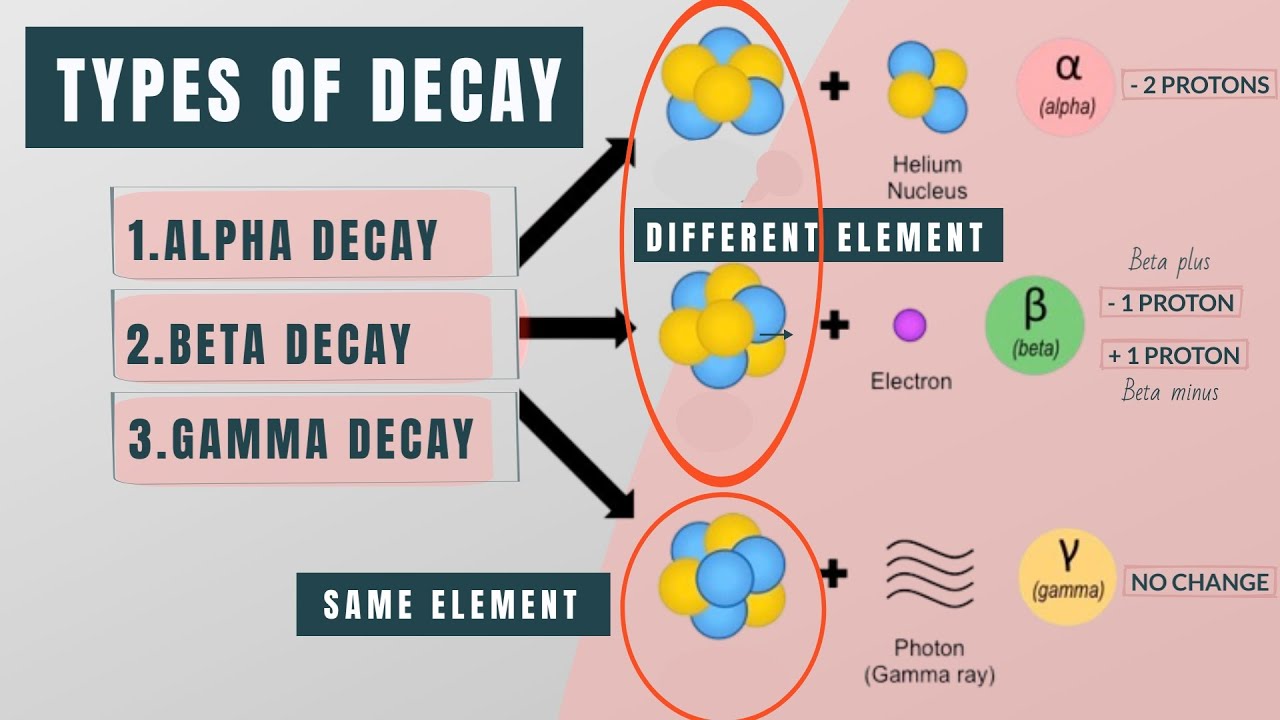
-Alpha decay is a form of radioactive decay where an unstable nucleus emits an alpha particle, which is a helium nucleus (2 protons + 2 neutrons). This changes the parent nucleus into a new daughter nucleus, resulting in transmutation to a new element.
What is beta decay?
-Beta decay is radioactive decay where a neutron converts into a proton, emitting an electron and an antineutrino in the process. This causes the nucleus to change from one element to another, but no nucleons are emitted like with alpha decay.
What causes gamma decay?
-Gamma decay occurs when an excited nucleus transitions to a lower energy state by emitting a high-energy gamma ray photon. No transmutation occurs, the nucleus just releases the excess energy as light.
How do binding energies per nucleon change across elements?
-Binding energies per nucleon increase up to around iron, then decrease for heavier elements. So iron nuclei have the highest binding energy per nucleon, making them the most stable.
Why do large nuclei require more neutrons?
-Large nuclei with many protons have stronger electric repulsion forces that push protons apart. Extra neutrons help overcome this repulsion via the strong nuclear force, stabilizing heavier nuclei.
What are the 4 fundamental forces of nature?
-The 4 fundamental forces of nature are the strong force, weak force, electromagnetic force, and gravitational force. The strong force binds nucleons, the weak force governs radioactive decay, electromagnetism controls chemistry, and gravity controls motion on a large scale.
How was radioactivity discovered?
-Radioactivity was discovered in 1896 by Henri Becquerel when photographic plates were exposed by uranium ore even when covered by dark paper, showing some radiation could penetrate the cover.
Outlines
🧬 Basic Nuclear Physics Concepts
Introduces key concepts in nuclear physics including atomic number, mass number, nuclear notation, binding energy, and mass-energy equivalence. Also discusses the strong and weak nuclear forces that hold the nucleus together and facilitate radioactive decay.
😵💫 Three Types of Radioactive Decay
Describes the three major types of radioactive decay - alpha, beta, and gamma. Explains the particles emitted in each type of decay, the transmutation process, penetrating power, and decay causes. Alpha decay involves helium nuclei, beta decay electrons, and gamma decay high-energy photons.
🎥 Video Production Credits
Lists the studios, creative team, and sponsors involved in producing the Crash Course Physics video.
Mindmap
Keywords
💡Nucleus
💡Proton
💡Neutron
💡Binding Energy
💡Strong Nuclear Force
💡Radioactive Decay
💡Alpha Decay
💡Beta Decay
💡Gamma Decay
💡Mass-Energy Equivalence
Highlights
The study found a significant increase in math test scores for students using the new teaching method
Professor Smith introduced an innovative approach to calculus using real-world examples and applications
The research contributes new theoretical models for understanding complex economic systems
Dr. Lee's work offers important practical applications for improving public health outcomes
This discovery challenges long-held assumptions about the origins of this ancient civilization
The study provides new insights into consumer psychology and shopping behaviors
This novel polymer material may enable lighter, more durable materials for transportation
Professor Wilson put forth an innovative interpretation of 16th century paintings
Dr. Patel's algorithm enables much faster processing of large datasets
The new framework provides a more nuanced view of geopolitical relationships
This archaeological finding provides evidence of trade routes connecting ancient civilizations
Dr. Cho's model offers predictive capabilities that may save lives during epidemics
The study reveals the importance of early childhood education for lifelong development
Professor Zhou's theory holds promise for significantly enhancing computing capabilities
This breakthrough in nanotechnology may enable the next generation of microprocessors
Transcripts
Browse More Related Video
Different types of decay | Alpha vs. Beta vs. Gamma decay | Visual Explanation

Half-Life and Radioactive Decay

Fission VS Fusion

Nuclear Fission: The Basics
Nuclear Chemistry Part 2 - Fusion and Fission: Crash Course Chemistry #39

Nuclear Binding Energy Per Nucleon & Mass Defect Problems - Nuclear Chemistry
5.0 / 5 (0 votes)
Thanks for rating: