Chemistry | Chemical equilibrium | Le Chartelie's Principle
TLDRThe video script is an engaging lecture on chemical equilibrium, focusing on Le Chatelier's Principle. It explains that chemical equilibrium is a state where the rate of the forward reaction equals the rate of the reverse reaction. The lecturer identifies three key factors affecting this equilibrium: concentration, temperature, and pressure. Using the seesaw model, the script illustrates how changes in these factors cause the equilibrium to shift, either favoring the forward or reverse reaction to counteract the disturbance. The principle is applied to examples, including the formation of ammonia from nitrogen and hydrogen, and the decomposition of calcium carbonate. The lecture concludes with a promise to apply these concepts to exam situations in a future video, encouraging viewers to engage with questions or feedback.
Takeaways
- 🌟 Chemical equilibrium is a state where the rate of the forward reaction equals the rate of the reverse reaction.
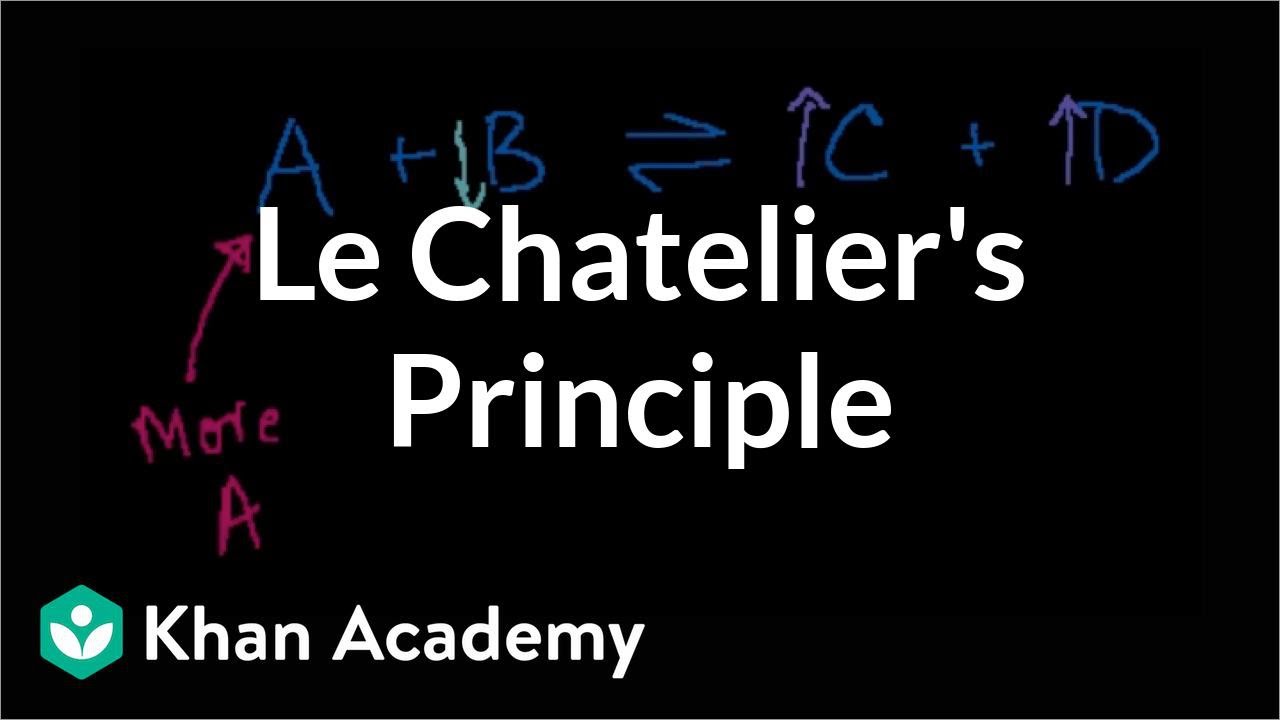
- ⚖️ Le Chatelier's Principle states that if a change is made to a system at equilibrium, the system will adjust to counteract the change and a new equilibrium will be established.
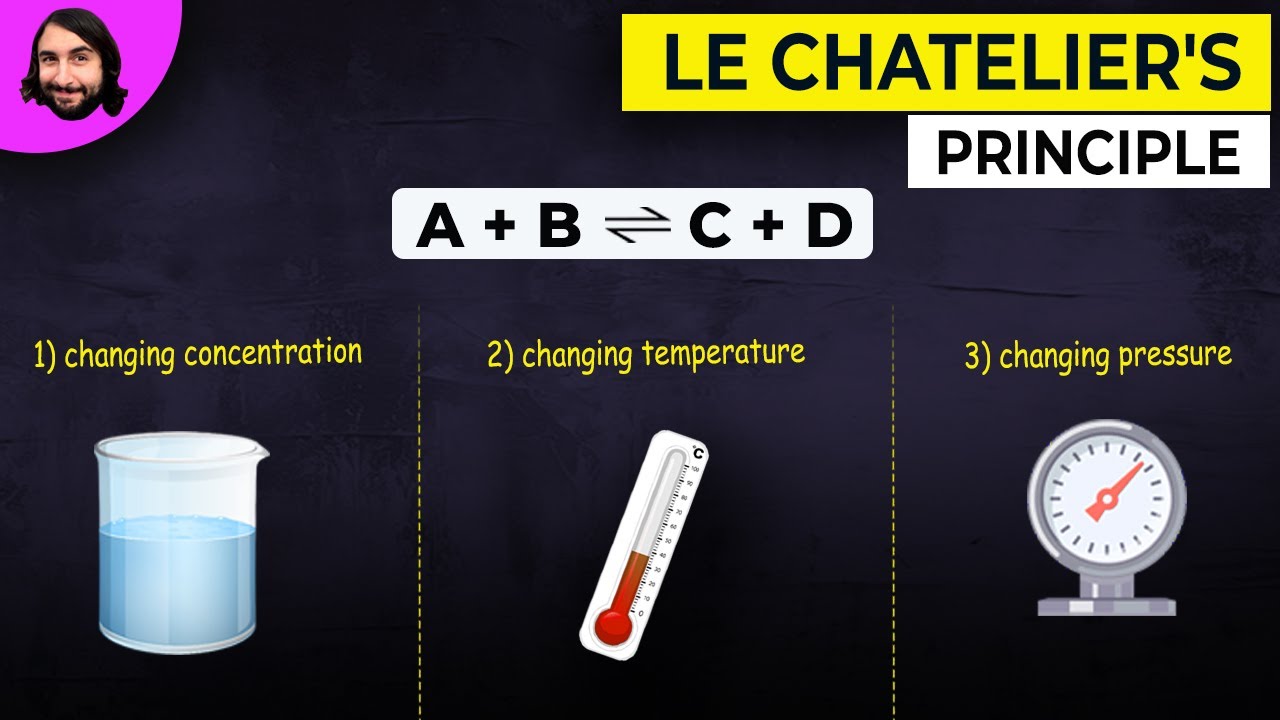
- 🔍 Three main factors affect chemical equilibrium: concentration, temperature, and pressure.
- 🧪 Concentration changes affect the equilibrium of substances in gaseous or aqueous states, not solids or pure liquids.
- ♨️ An increase in temperature favors the endothermic reaction (absorbs heat), while a decrease in temperature favors the exothermic reaction (releases heat).
- 📈 When pressure is increased, the equilibrium shifts toward the side with fewer moles of gas; when pressure is decreased, it shifts toward the side with more moles of gas.
- 🔁 Adding a reactant increases the concentration and shifts the equilibrium towards the products, while removing a reactant shifts it back towards the reactants.
- 🔥 For exothermic reactions (ΔH < 0), increasing temperature shifts the equilibrium towards the reactants, and decreasing temperature shifts it towards the products.
- 🧊 For endothermic reactions (ΔH > 0), increasing temperature shifts the equilibrium towards the products, and decreasing temperature shifts it towards the reactants.
- 🏗️ A catalyst does not affect the position of equilibrium because it speeds up both the forward and reverse reactions equally.
- 📚 Understanding and applying Le Chatelier's Principle is crucial for predicting shifts in chemical equilibrium in response to changes in concentration, temperature, or pressure.
Q & A
What is chemical equilibrium?
-Chemical equilibrium is a state in which the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products.
What are the three factors that affect chemical equilibrium?
-The three factors that affect chemical equilibrium are concentration, temperature, and pressure.
How does Le Chatelier's principle explain the effect of concentration changes on equilibrium?
-Le Chatelier's principle states that if a change is made to the concentration of reactants or products, the equilibrium will shift in a direction that counteracts the change, either by producing more of the substance that was removed or by consuming more of the substance that was added.
What is an endothermic reaction?
-An endothermic reaction is a chemical reaction in which energy, usually in the form of heat, is absorbed from the surroundings. In terms of enthalpy change (ΔH), it is represented as ΔH > 0.
What is an exothermic reaction?
-An exothermic reaction is a chemical reaction in which energy, usually in the form of heat, is released to the surroundings. In terms of enthalpy change (ΔH), it is represented as ΔH < 0.
How does temperature affect the equilibrium of an exothermic reaction?
-According to Le Chatelier's principle, increasing the temperature of an exothermic reaction (ΔH < 0) will favor the reverse reaction, which is endothermic. This results in a decrease in the amount of products and an increase in the amount of reactants.
How does pressure affect the equilibrium of a reaction involving gases?
-Pressure affects the equilibrium of a reaction involving gases by shifting the equilibrium towards the side with fewer moles of gas when the pressure is increased. Conversely, decreasing the pressure will shift the equilibrium towards the side with more moles of gas.
Why does Le Chatelier's principle not consider catalysts or surface area changes?
-Le Chatelier's principle does not consider catalysts or changes in surface area because a catalyst affects both the forward and reverse reactions equally, thus not disturbing the equilibrium. Surface area changes affect the rate of heterogeneous reactions, which typically do not occur in closed systems where Le Chatelier's principle is applied.
What is the significance of the seesaw model used in the script to explain Le Chatelier's principle?
-The seesaw model is used as an analogy to visualize how equilibrium shifts in response to changes in concentration, temperature, or pressure. It helps to illustrate the concept that equilibrium will adjust in a way that counteracts the imposed change, much like a seesaw would balance out after adding or removing weight.
How does the equilibrium shift if the concentration of a reactant is increased in a closed system?
-If the concentration of a reactant is increased in a closed system, the equilibrium will shift towards the side of the products to consume the excess reactant, favoring the forward reaction.
What happens to the amount of ammonia (NH3) at equilibrium if the concentration of nitrogen is decreased?
-If the concentration of nitrogen is decreased, the equilibrium will shift towards the reverse reaction to produce more nitrogen. As a result, the amount of ammonia (NH3) at equilibrium will decrease.
How does the equilibrium shift if the pressure is increased for a reaction where the left side has more moles of gas than the right side?
-If the pressure is increased for a reaction where the left side has more moles of gas than the right side, the equilibrium will shift towards the side with fewer moles of gas, which is the right side in this case.
Outlines
😀 Introduction to Chemical Equilibrium and Le Chatelier's Principle
The video begins with a greeting and an introduction to the topic of chemical equilibrium. The presenter explains that chemical equilibrium is a state where the rate of the forward reaction equals the rate of the reverse reaction. They also introduce the factors that affect chemical equilibrium: concentration, temperature, and pressure. The presenter emphasizes the importance of understanding how these factors influence equilibrium and introduces Le Chatelier's principle, which states that if a change is applied to a system at equilibrium, the system will adjust to counteract the change and establish a new equilibrium.
🔍 Applying Le Chatelier's Principle with a Seesaw Model
The presenter uses a seesaw model to illustrate Le Chatelier's principle. They describe a scenario where adding more weight (or 'children') to one side of the seesaw (representing an increase in concentration) causes an imbalance. To restore equilibrium, a large ball (representing the system's response) is moved to the opposite side. This model is used to show that when a system at equilibrium is disturbed, it will shift to counteract the disturbance. The video also clarifies that catalysts and surface area are not included in this discussion because they affect different types of reactions and do not disturb chemical equilibrium in closed systems.
🤔 Shifts in Equilibrium due to Changes in Concentration
The video discusses how changes in the concentration of reactants or products affect the equilibrium of a closed system. It uses the example of the reaction between nitrogen and hydrogen to form ammonia. The presenter explains that adding more nitrogen will disturb the equilibrium, causing the system to shift towards the right to consume the added nitrogen, thereby increasing the amount of ammonia produced. Conversely, removing nitrogen from the system will cause the equilibrium to shift to the left, favoring the reverse reaction and decreasing the amount of ammonia.
🌡️ The Effect of Temperature Changes on Equilibrium
The presenter explains the impact of temperature on chemical equilibrium, differentiating between endothermic and exothermic reactions. They use the mnemonic of Pac-Man to help remember that an increase in temperature favors the endothermic reaction (where heat is on the right side of the reaction), and a decrease in temperature favors the exothermic reaction. The video provides an example of how increasing temperature affects the yield in an exothermic reaction, causing the equilibrium to shift to the left and decrease the amount of products formed.
📉 Impact of Pressure on Equilibrium in Gas Reactions
The video addresses how pressure affects the equilibrium of gaseous reactions. It explains that pressure changes only impact the equilibrium if there is a difference in the number of moles of gas between reactants and products. An increase in pressure favors the side with fewer moles of gas, while a decrease in pressure favors the side with more moles of gas. The presenter uses the example of the reaction between nitrogen and hydrogen to form ammonia, illustrating that increasing pressure would shift the equilibrium to the right, increasing the amount of ammonia produced.
🔧 Applying Le Chatelier's Principle to Exam Questions
The presenter concludes the video by summarizing the application of Le Chatelier's principle to the three factors affecting equilibrium: concentration, temperature, and pressure. They remind viewers that these factors only affect gases and aqueous solutions, not solids or liquids. The video promises to show how these concepts are applied in exam situations in a future video. The presenter encourages viewers to ask questions or provide feedback via email and to comment on the video if they found the illustrations helpful.
Mindmap
Keywords
💡Chemical Equilibrium
💡Le Chatelier's Principle
💡Concentration
💡Temperature
💡Pressure
💡Endothermic Reaction
💡Exothermic Reaction
💡Seesaw Model
💡Dynamic Equilibrium
💡Gaseous State
💡Moles of Gas
Highlights
Chemical equilibrium is defined as the state where the rate of the forward reaction equals the rate of the reverse reaction.
Three factors affect the state of chemical equilibrium: concentration, temperature, and pressure.
Le Chatelier's Principle states that if one of the equilibrium affecting factors changes, the equilibrium will adjust to counteract the change.
Concentration affects chemical equilibrium only for substances in gaseous or aqueous form, not for solids or pure liquids.
An increase in the concentration of a reactant will shift the equilibrium to favor the forward reaction, forming more products.
A decrease in the concentration of a reactant will shift the equilibrium towards the reverse reaction, breaking down more products into reactants.
Temperature changes affect equilibrium differently for endothermic and exothermic reactions.
An increase in temperature favors the endothermic reaction, shifting the equilibrium towards the reactants that absorb heat.
A decrease in temperature favors the exothermic reaction, shifting the equilibrium towards the products that release heat.
Pressure changes only affect the chemical equilibrium of gaseous reactions, not solids or liquids.
Increasing pressure favors the side of the reaction with fewer moles of gas, shifting the equilibrium towards that side.
Decreasing pressure favors the side with more moles of gas, shifting the equilibrium towards the side with more gaseous reactants.
Catalysts do not affect the position of equilibrium as they equally favor both forward and reverse reactions.
Le Chatelier's Principle is used to predict the direction of equilibrium shifts in response to changes in concentration, temperature, and pressure.
The seesaw model is used as an analogy to help visualize and understand shifts in chemical equilibrium.
The direction of the shift in equilibrium depends on whether the system is adding or removing reactants or products.
Le Chatelier's Principle is applicable to closed systems where no reactants or products can escape.
The concept of equilibrium shift is illustrated by adding or removing 'children' or a 'red ball' on a seesaw to represent changes in concentration or pressure.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: