Le Chatelier's principle | Chemical equilibrium | Chemistry | Khan Academy
TLDRThe video script discusses the concept of dynamic equilibrium in chemical reactions, where the forward and reverse reaction rates are equal, resulting in constant concentrations of reactants and products. It explores how adding more of a reactant or product, changing temperature, or applying pressure can shift the equilibrium according to Le Chatelier's Principle, which states that a system at equilibrium will adjust to minimize the effect of the change imposed on it. The example of the Haber process is used to illustrate how pressure affects the formation of ammonia from nitrogen and hydrogen.
Takeaways
- 🌟 Dynamic equilibrium occurs when the forward and reverse reaction rates are equal, not necessarily meaning equal concentrations of reactants and products.
- ⚖️ Adding more of a reactant (like A) to a system at equilibrium will shift the balance towards the products (C and D), consuming more of the other reactant (B) and increasing the concentration of the products.
- 🔄 If more A and B are added, the reaction will shift more towards the forward direction, producing more C and D.
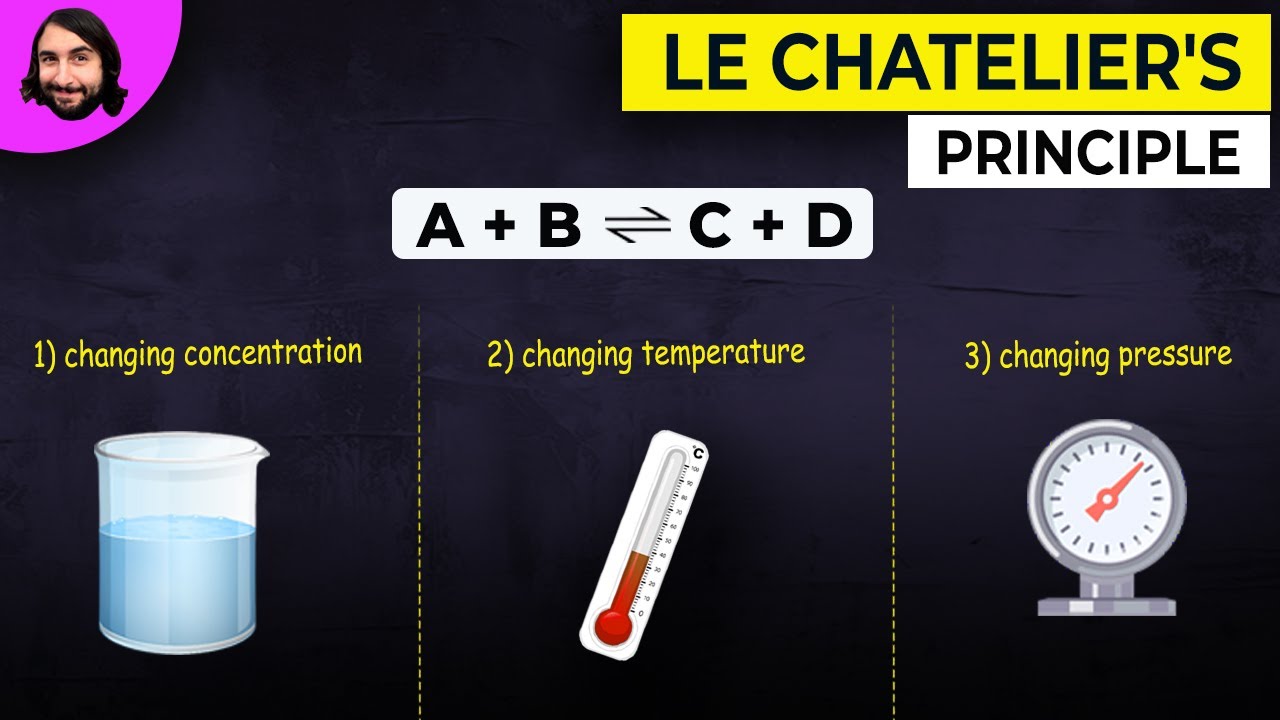
- 📈 Le Chatelier's Principle states that when a reaction at equilibrium is stressed (by changes in concentration, pressure, or temperature), the reaction will adjust to relieve that stress by favoring one side of the reaction.
- 🔥 Adding heat to a system favors the endothermic direction (the direction that absorbs heat), increasing the concentration of the products that require heat for their formation.
- 🌡️ Removing heat from a system will favor the exothermic direction (the direction that releases heat), shifting the equilibrium towards the reactants.
- 💥 Applying pressure to a system favors the side of the reaction with fewer gas molecules, reducing the number of collisions and easing the pressure.
- 📊 The equilibrium constant remains constant at a given temperature, and it can be used to calculate the new equilibrium concentrations when the system is stressed.
- 🔄 Changes in the concentrations of reactants and products due to stress on the system are temporary until a new equilibrium is established.
- 🧪 The Haber process, which combines nitrogen and hydrogen to form ammonia, is an example of how pressure can affect the equilibrium of a reaction with gaseous reactants and products.
- 📚 Understanding Le Chatelier's Principle is crucial for predicting how changes in conditions will affect chemical equilibria and can be applied to various types of reactions and stressors.
Q & A
What is dynamic equilibrium in the context of chemical reactions?
-Dynamic equilibrium refers to a state in a chemical reaction where the forward and reverse reactions occur at the same rate, resulting in constant concentrations of reactants and products, even though they are not changing anymore.
What happens when you add more of a reactant (like molecule A) to a system at equilibrium?
-When more of a reactant is added to a system at equilibrium, the forward reaction rate initially increases, consuming more of the other reactant (like molecule B) and producing more of the products (like molecules C and D). Eventually, a new equilibrium is established with higher concentrations of the added reactant and its corresponding products.
How does the concentration of reactants and products change when additional A is introduced to the system?
-Upon introducing more A, the concentration of A increases, B decreases slightly due to increased consumption in the forward reaction, and the concentrations of C and D increase as they are produced more frequently in the now faster forward reaction.
What is Le Chatelier's principle and how does it apply to chemical reactions at equilibrium?
-Le Chatelier's principle states that when a reaction at equilibrium is stressed by changes in conditions such as concentration, temperature, or pressure, the reaction will shift in a direction that alleviates the stress. This principle helps predict how changes in external conditions will affect the position of equilibrium.
How does adding heat to a system affect the equilibrium of a reaction?
-Adding heat to a system favors the direction of the reaction that absorbs heat. If the forward reaction is endothermic (absorbs heat), the system will shift to produce more products. If the reverse reaction is endothermic, the system will shift to produce more reactants.
What is the effect of removing heat from a system at equilibrium?
-Removing heat from a system at equilibrium favors the direction of the reaction that releases heat. The system will shift towards the exothermic direction (the direction that gives off heat), resulting in a decrease in the concentration of products and an increase in reactants.
How does pressure affect the position of equilibrium in reactions involving gases?
-Pressure affects the position of equilibrium in gaseous reactions by favoring the side with fewer moles of gas. Increasing pressure will shift the equilibrium towards the side that reduces the number of gas molecules, thereby relieving the stress caused by increased pressure.
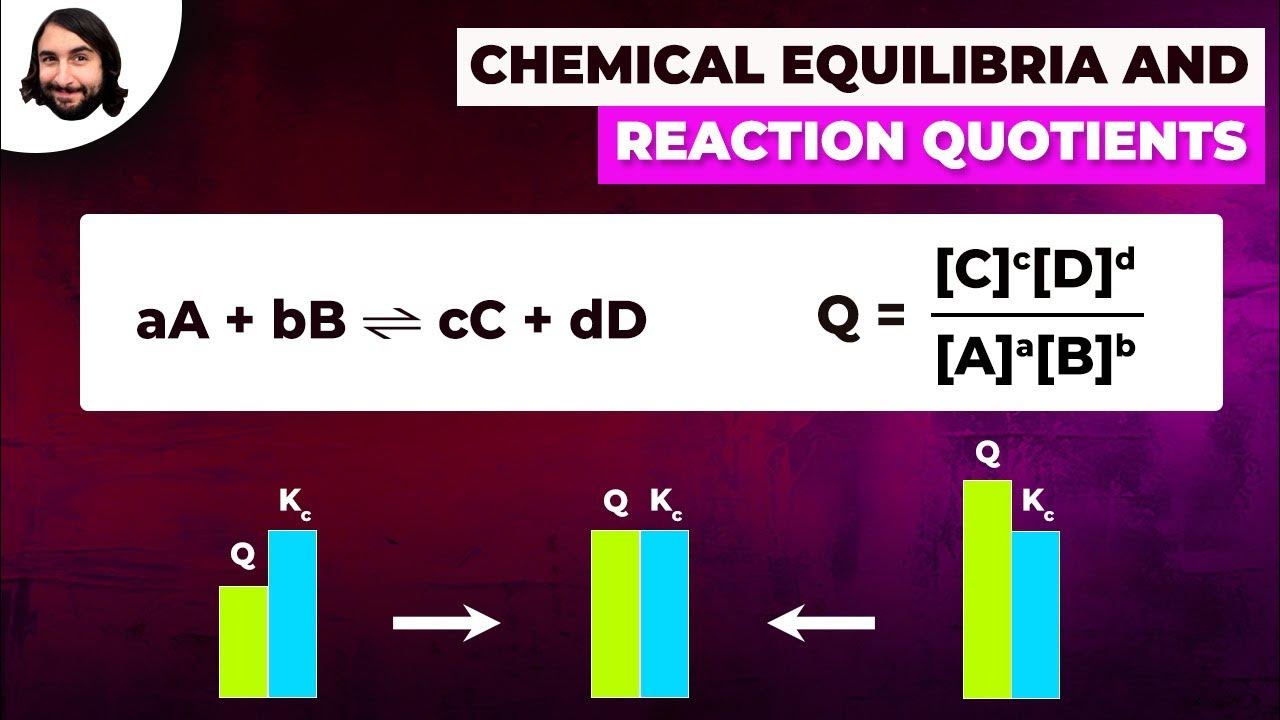
What is the relationship between the equilibrium constant and the concentrations of reactants and products at equilibrium?
-The equilibrium constant (K) is the ratio of the product of the concentrations of the products raised to their stoichiometric coefficients to the product of the concentrations of the reactants raised to their stoichiometric coefficients. At equilibrium, this ratio remains constant, even if the individual concentrations change due to the addition or removal of reactants or products.
How does the equilibrium constant (K) change when conditions such as concentration are altered?
-The equilibrium constant (K) is a fixed value at a given temperature and does not change when conditions such as concentration are altered. However, the actual concentrations of reactants and products at equilibrium will adjust to maintain the value of K under the new conditions.
Can you provide an example of how Le Chatelier's principle applies to the Haber process?
-In the Haber process, nitrogen gas reacts with hydrogen gas to form ammonia gas. According to Le Chatelier's principle, if pressure is applied to this system, the reaction will shift to produce fewer gas molecules, thereby relieving the stress of increased pressure. This means the reaction will favor the production of ammonia, which has fewer moles of gas (2 moles) compared to the reactants (1 mole of nitrogen and 3 moles of hydrogen, totaling 4 moles).
What is the significance of understanding Le Chatelier's principle in the study of chemistry?
-Understanding Le Chatelier's principle is crucial in chemistry as it provides a fundamental concept to predict and explain how changes in external conditions such as concentration, temperature, and pressure will affect chemical equilibria. This knowledge is essential for controlling and optimizing chemical processes in industries, laboratories, and environmental management.
Outlines
🌟 Dynamic Equilibrium and Le Chatelier's Principle
This paragraph introduces the concept of dynamic equilibrium in chemical reactions, where the forward and reverse reaction rates are equal, resulting in constant concentrations of reactants and products. It explains how adding more of a reactant (molecule A) to the system can shift the equilibrium, causing an increase in the concentration of products (molecules C and D) and a decrease in the concentration of the other reactant (molecule B). The paragraph also introduces Le Chatelier's principle, which states that when a system at equilibrium is stressed (e.g., by adding more reactant), the reaction will adjust to relieve the stress by favoring one direction of the reaction.
🔥 The Effect of Adding Heat on Chemical Equilibrium
This paragraph discusses the impact of heat on chemical equilibrium. It explains how adding heat to a system can stress the reaction and cause it to shift in the direction that consumes the added heat. The example given involves a reaction where heat is a reactant, and adding more heat will drive the reaction forward, consuming more of the reactants and producing more products. Conversely, removing heat from the system will favor the reverse reaction, which produces heat and reduces the concentration of the products formed.
📈 Pressure and Its Influence on Equilibrium
This paragraph explores the effect of pressure on chemical equilibrium, specifically focusing on the Haber process, which produces ammonia from nitrogen and hydrogen gases. It explains that applying pressure to a system can cause the reaction to shift in the direction that results in fewer gas molecules, thereby relieving the stress of increased pressure. This is because fewer molecules mean less collision and less resistance to compression. The paragraph uses the ideal gas law (PV=nRT) to illustrate how changes in pressure can affect the number of molecules in a given volume, and thus the equilibrium position.
📊 Equilibrium Constants and Reaction Adjustments
The final paragraph ties together the concepts of equilibrium constants and Le Chatelier's principle. It provides a hypothetical reaction with initial equilibrium concentrations for reactants A and B and product C. The paragraph then demonstrates how adding more A to the system changes the equilibrium concentrations of A, B, and C, while the equilibrium constant remains constant. The example shows the mathematical relationship between the concentrations and the equilibrium constant, and how the system adjusts to maintain this constant when the conditions change.
Mindmap
Keywords
💡Dynamic Equilibrium
💡Equilibrium Constant
💡Le Chatelier's Principle
💡Concentration
💡Forward Reaction
💡Reverse Reaction
💡Stress on a Reaction
💡Moles
💡Heat
💡Pressure
💡Haber Process
Highlights
Dynamic equilibrium is when the forward and backward reaction rates are equal, but concentrations of the reactants and products can be different.
Adding more of a reactant (A) to a system at equilibrium will shift the balance towards the products (C and D), consuming more of the other reactant (B) and increasing the concentration of the products.
The concentrations at equilibrium are constant, but they are not necessarily equal, reflecting the rates of the forward and reverse reactions.
Le Chatelier's principle states that when a reaction at equilibrium is stressed, the reaction will favor the side that relieves the stress.
Stressing a reaction can involve adding more reactants, heat, or pressure, causing the reaction to shift in the direction that counteracts the change.
If heat is added to a reaction that requires heat to proceed in the forward direction, the concentration of the products will increase.
Removing heat from a reaction will favor the reverse reaction, increasing the concentration of the reactants.
Applying pressure to a system favors the side of the reaction with fewer gas molecules, reducing the number of particles and thus relieving the stress.
The Haber process, producing ammonia from nitrogen and hydrogen, is an example of a reaction where pressure can affect the equilibrium.
The equilibrium constant remains constant regardless of the changes in concentration as the system adjusts to a new equilibrium.
The mathematical approach to equilibrium problems involves understanding and applying the equilibrium constant expression.
The reaction 2A + B in equilibrium with C illustrates how changes in concentration of one reactant can be calculated after a disturbance to the system.
When more A is added to the system, the new equilibrium concentrations of A, B, and C can be determined using the equilibrium constant and stoichiometry.
The concept of kinetic equilibrium and Le Chatelier's principle are consistent with each other and can be used to predict the behavior of reactions under changing conditions.
The system will naturally move to counteract added stress, such as adding more reactants or changing temperature, by shifting the equilibrium position.
Understanding the effects of stress on a reaction at equilibrium is crucial for controlling chemical processes in industry and research.
The example of nitrogen and hydrogen gases in equilibrium with ammonia gas demonstrates how pressure can shift the equilibrium in the Haber process.
The equilibrium constant K is calculated as the product of the concentration of the products divided by the concentration of the reactants raised to their stoichiometric coefficients.
When a system is at equilibrium and then disturbed, it will adjust to reach a new equilibrium state that relieves the stress.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: