The Equilibrium Constant
TLDRIn this chemistry essentials video, Mr. Andersen explains the concept of the equilibrium constant (K), which is crucial for understanding reversible reactions. He uses the historical example of phosgene, a chemical weapon from World War I, to illustrate how reactants and products can be converted into each other. The video demonstrates how a large K value indicates a reaction that favors the formation of products, while a small K value suggests a reaction that favors reactants. Mr. Andersen also discusses how temperature changes can affect K values, shifting the balance between reactants and products. He concludes by emphasizing the importance of K values in predicting the outcome of chemical reactions at equilibrium, providing a clear and informative overview of a complex topic.
Takeaways
- 🧪 The equilibrium constant (K) is used to determine whether there are more reactants or products in a reversible chemical reaction at equilibrium.
- 🌟 Phosgene, a chemical weapon used in WWI, is an example of a substance formed through a reversible reaction between carbon monoxide and chlorine gas.
- 📉 At standard temperature and pressure, a large K value for a reaction indicates that more products are formed than reactants.
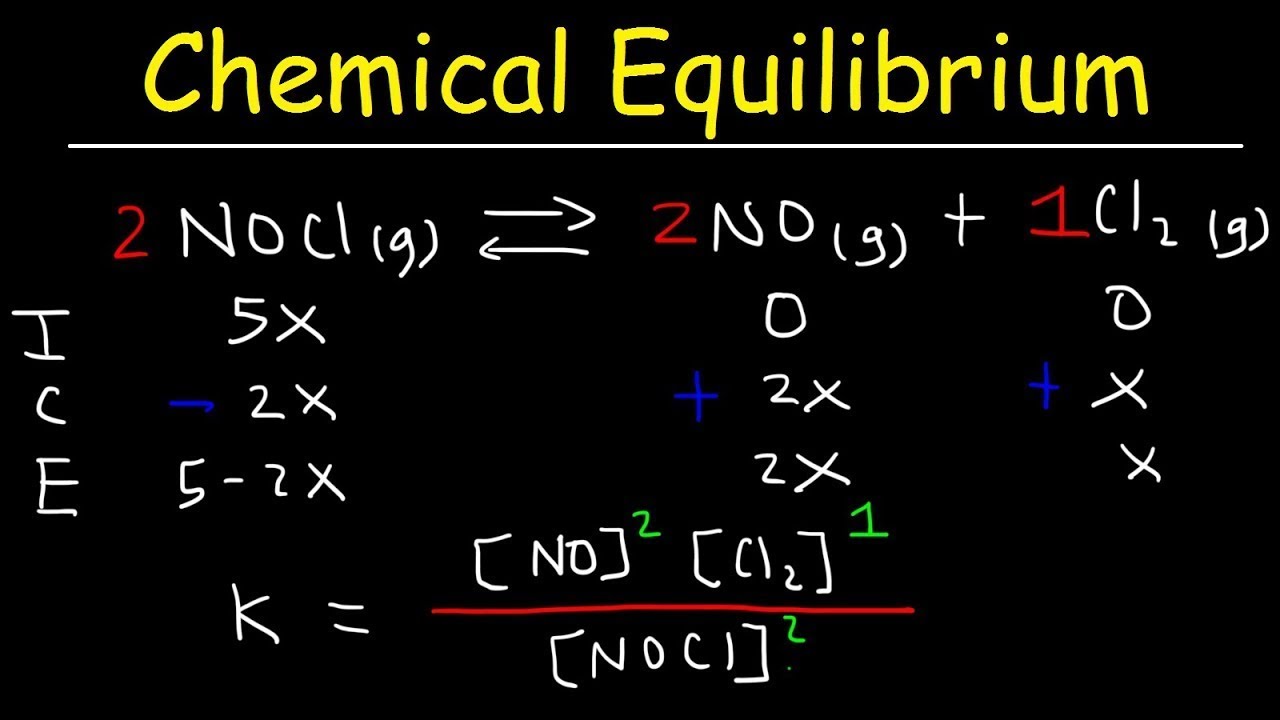
- 🔍 K value is calculated by dividing the concentration of products by the concentration of reactants, each raised to the power of their stoichiometric coefficients.
- 📊 In a PHET simulation model, the K-ometer visually represents the ratio of products to reactants, with changes in the ratio reflected in the K value.
- ⚖️ For complex reactions, the general formula for K includes the concentration of each product and reactant raised to their respective mole powers.
- 🧪 The equilibrium constant for a reversible reaction like the formation of phosgene from CO and Cl2 can be calculated and is represented as the concentration of products over reactants.
- 🔥 The K value can be influenced by temperature changes; an increase in temperature can shift the equilibrium towards the products or reactants depending on the reaction.
- ⚠️ A K value greater than 1 indicates that at equilibrium, there are more products than reactants, while a K value less than 1 indicates the opposite.
- 🌡️ For the reaction converting nitrogen and oxygen into nitrogen oxide, a high temperature (like a lightning strike) is required to significantly form nitrogen oxide from the reactants.
- 📚 Understanding K values allows chemists to predict the composition of reactants and products at equilibrium, which is crucial for various chemical processes and industrial applications.
Q & A
What is the historical context of phosgene mentioned in the video?
-Phosgene was a chemical weapon used during World War I that resulted in the death of a significant number of people.
How is phosgene created in the context of the video?
-Phosgene is created through a reversible reaction by mixing carbon monoxide and chlorine gas.
What is the significance of phosgene being colorless and having a smell like hay?
-Being colorless and having a distinct smell made it difficult to detect, which was a critical factor during World War II when people needed to be warned of its potential presence.
What does the equilibrium constant (K value) indicate in a chemical reaction?
-The equilibrium constant (K value) indicates whether at equilibrium there are more reactants or more products in a reversible chemical reaction.
What does a large K value at standard temperature and pressure imply for the phosgene reaction?
-A large K value implies that the reaction will move towards the product side, resulting in a greater amount of phosgene compared to the reactants.
How does the K-ometer in the PHET simulation measure the K value?
-The K-ometer measures the K value by calculating the ratio of the number of product molecules to the number of reactant molecules.
What happens to the K value when the simulation is tipped towards producing more products?
-When the simulation is tipped towards producing more products, the K value increases, indicating a higher concentration of products relative to reactants.
How does the equilibrium constant expression for a reaction with multiple reactants and products look?
-The equilibrium constant expression is written with the concentrations of the products raised to the power of their respective stoichiometric coefficients in the numerator, and the concentrations of the reactants raised to the power of their respective stoichiometric coefficients in the denominator.
What is the equilibrium constant for the phosgene reaction at standard temperature and pressure?
-At standard temperature and pressure, the equilibrium constant for the phosgene reaction is 4.57 times 10 to the 9th.
How does temperature affect the K value in a chemical reaction?
-Changing the temperature can alter the K value. An increase in temperature generally shifts the equilibrium towards the endothermic direction (either towards products or reactants, depending on the reaction), while a decrease in temperature will shift it towards the exothermic direction.
What does a small K value signify in terms of reactants and products at equilibrium?
-A small K value signifies that there are more reactants than products at equilibrium, indicating that the reaction favors the reactant side.
How can you predict the side on which the chemical reaction will favor at equilibrium using K values?
-If the K value is greater than 1, the reaction favors the product side with more products at equilibrium. If the K value is less than 1, it favors the reactant side with more reactants at equilibrium.
Outlines
🧪 Understanding the Equilibrium Constant and Phosgene Reaction
This paragraph introduces the concept of the equilibrium constant (K) in the context of a chemical reaction that produces phosgene, a chemical weapon used during World War I. The reaction is reversible, involving carbon monoxide and chlorine gas to form phosgene. The equilibrium constant is crucial to determine whether there are more reactants or products at equilibrium. The video uses a PHET simulation to visually demonstrate how the K value changes as the reaction shifts from reactants to products and vice versa. The K value is calculated as the ratio of products to reactants, and it is represented by the concentrations of the products and reactants raised to the power of their respective moles. The example of phosgene's equilibrium constant is provided, showing how it is calculated and what a high K value implies about the prevalence of products over reactants. The paragraph also touches on how temperature can affect the K value and shift the equilibrium, using the example of nitrogen and oxygen gases converting to nitrogen oxide.
🔥 Effect of Temperature on Equilibrium and the Role of Energy
The second paragraph delves into the effect of temperature on the equilibrium constant. It explains that increasing the temperature, such as during a lightning strike, can increase the K value and push the reaction towards the formation of more products. The paragraph uses the example of nitrogen and oxygen gases, which do not readily convert to nitrogen oxide under standard conditions due to a small K value. However, with sufficient energy, such as from lightning, the reaction is more likely to occur. The summary emphasizes that a K value greater than 1 indicates a reaction that favors products, while a K value less than 1 suggests a reaction that favors reactants. The paragraph concludes by encouraging viewers to use the values of K to predict the composition of chemicals at equilibrium.
Mindmap
Keywords
💡Equilibrium Constant (K)
💡Phosgene
💡Reversible Reaction
💡Standard Temperature and Pressure (STP)
💡Stoichiometric Coefficients
💡Concentration
💡Chemical Equilibrium
💡World War I Poster
💡Colorless
💡Hay-like Smell
💡Temperature Effect on K
Highlights
Mr. Andersen discusses the equilibrium constant in the context of phosgene, a chemical weapon used in World War I.
Phosgene is produced through a reversible reaction involving carbon monoxide and chlorine gas.
The equilibrium constant (K) is used to determine whether there are more reactants or products at equilibrium.
A large K value at standard temperature and pressure indicates a shift towards the product side in a reversible reaction.
The K value is calculated as the ratio of the number of products to reactants at equilibrium.
A PHET simulation is used to demonstrate how reversible reactions work and how K value changes with the shift of molecules.
The equilibrium constant is represented by the concentration of products divided by the concentration of reactants, each raised to the power of their respective moles.
For the phosgene reaction, the K value at standard temperature and pressure is very large, indicating a high concentration of phosgene.
The K value for phosgene at standard temperature and pressure is 4.57 times 10 to the 9th, indicating a strong formation of phosgene.
Changing the temperature can alter the K value, causing a shift in the equilibrium position.
At 200 degrees Celsius, the K value for the phosgene reaction decreases, favoring reactants over products.
The formation of nitrogen oxide from nitrogen and oxygen gases is represented by a small K value, indicating a low concentration of products at equilibrium.
An increase in temperature, such as from a lightning strike, can increase the K value and shift the equilibrium towards the product side.
The equilibrium constant helps predict the concentration of products and reactants at equilibrium.
If K is greater than 1, the reaction favors products; if K is less than 1, it favors reactants.
Understanding K values is crucial for predicting chemical concentrations at equilibrium.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: