Nuclear Chemistry Part 2 - Fusion and Fission: Crash Course Chemistry #39
TLDRThis chemistry video explains nuclear stability and binding energy using Einstein's famous equation E=mc^2. It covers fission reactions, where large unstable nuclei split apart, and fusion reactions, where small nuclei join together. Fission reactions power nuclear plants but produce radioactive waste. Fusion reactions release more energy but require extremely high temperatures, making them difficult to control for power generation. The video challenges viewers to find new ways to better harness nuclear power while overcoming existing limitations.
Takeaways
- 😀 Stability in chemistry means nuclei staying together instead of breaking apart, releasing energy
- 😮 Binding energy, calculated using Einstein's E=mc^2 formula, determines nuclear stability
- 🔥 Fission splits large unstable nuclei into smaller, more stable pieces, releasing energy
- 💥Fusion joins smaller nuclei into a larger, more stable one, releasing even more energy
- ⚛️ Nuclear power comes from controlling fission reactions to produce usable heat
- ⚠️ Uncontrolled fission reactions lead to meltdowns from too much heat or explosions from chain reactions
- ❄️ Control rods and cooling water help regulate heat production from fission reactions
- ☢ Toxic radioactive waste from uncontrolled reactions lasts a long time
- 🚀 Fusion could provide abundant clean energy but requires immensely high temperatures and pressures
- 🧠 New ideas are needed to better harness nuclear energy safely for society's use
Q & A
What is binding energy and how is it related to nuclear chemistry?
-Binding energy is the energy required to hold the protons and neutrons of an atom's nucleus together. The binding energy of a nucleus is released when nucleons are removed, which is the basis of nuclear reactions and nuclear energy.
What is the mass defect of an atom and how does it relate to binding energy?
-The mass defect is the difference between the total mass of an atom's protons and neutrons and the actual mass of the nucleus. This 'missing mass' is present as binding energy holding the nucleus together. The larger the mass defect, the more stable the atom.
How does Einstein's formula E=mc^2 relate to nuclear chemistry?
-E=mc^2 shows the equivalence of mass and energy. The 'missing mass' in an atom's nucleus corresponds to its binding energy. By plugging the mass defect into Einstein's formula, we can calculate the nuclear binding energy released in nuclear reactions.
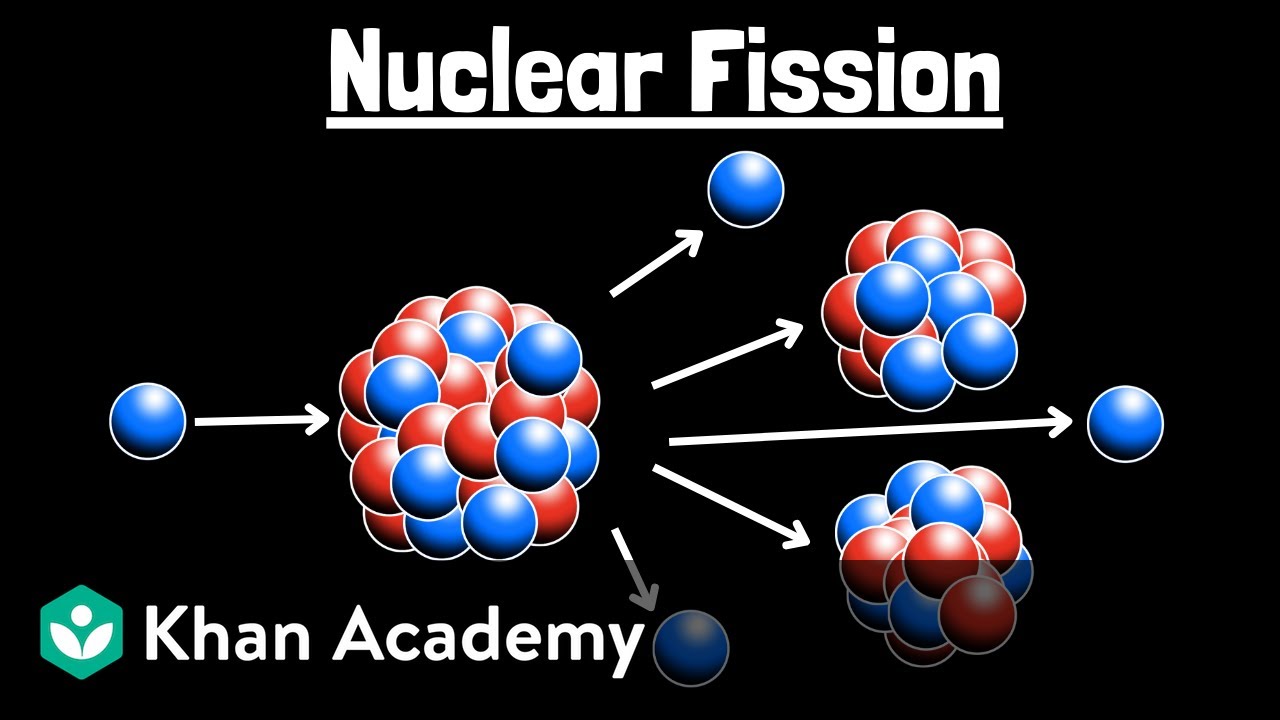
What is the difference between nuclear fission and fusion?
-Fission is the splitting of a heavy unstable nucleus into lighter nuclei, releasing energy. Fusion is the joining of two light nuclei into a heavier, more stable one, also releasing large amounts of energy.
How are nuclear chain reactions controlled in power plants?
-Control rods made of neutron-absorbing material are inserted between the fuel rods to slow down the neutrons and control the rate of fission reactions, preventing a runaway chain reaction.
Why is radioactive waste from fission reactions a problem?
-The unstable fission products continue to decay radioactively for a long time after the reaction, releasing dangerous radiation. Radioactive waste must be safely contained and stored long-term.
Why is fusion more difficult to achieve than fission?
-Fusion requires extremely high temperatures and pressures to overcome electrostatic repulsion between nuclei. The conditions are much harder to generate in a controlled way compared to fission.
How does the sun produce energy via nuclear fusion?
-The sun fuses hydrogen atoms into progressively heavier helium isotopes, releasing energy at each step. This fusion chain reaction provides the sun's enormous power.
Why can't we currently use fusion to generate electricity?
-We can't yet control or contain the extremely high temperatures and speeds required for fusion. So far it can only be used for uncontrolled explosive release of energy.
How might future discoveries advance nuclear chemistry?
-Possible future breakthroughs include: better fusion control for clean energy; more efficient fission reactions; improved radioactive waste storage; and new equations like E=mc^2 that reshape our understanding.
Outlines
🧪 Introducing Nuclear Stability and Mass-Energy Equivalence
This paragraph introduces the concept of nuclear stability, explaining that atomic nuclei strive for stability by getting rid of particles through radioactive decay. It defines stability as a nucleus remaining intact rather than breaking apart. Binding energy that holds nucleons together is equivalent to the energy released when a nucleon is removed, as described by Einstein's famous mass-energy equivalence formula E=mc2.
🔬 Explaining Fission and Fusion Nuclear Reactions
This paragraph distinguishes between the two types of nuclear reactions: fission, in which a large nucleus splits into smaller ones, and fusion, where two smaller nuclei join to form a larger one. In both cases, the resulting products are more stable, releasing energy. Elements react differently based on their position on a binding energy graph relative to iron-56.
🌞 Imagining Potential Applications of Nuclear Reactions
This closing paragraph suggests there are opportunities to explore new applications of nuclear reactions, like better utilizing radioactive materials, achieving fusion reactions, and harnessing nuclear power safely. It encourages viewers to build on the fundamentals covered in the video to make new discoveries in nuclear chemistry.
Mindmap
Keywords
💡Binding energy
💡Mass defect
💡Nuclear fission
💡Nuclear fusion
💡Chain reaction
💡Radioactive decay
💡Half-life
💡Control rods
💡Plasma
💡E=mc^2
Highlights
The framework provides an end-to-end approach for building AI systems that aligns data, models, and application design with social norms and values.
Values are operationalized by identifying potential harms, specifying formal constraints, and incorporating them into the optimization process.
The value alignment process involves stakeholders to represent diverse viewpoints and promotes transparency.
Formal verification methods help provide mathematical guarantees that implemented systems meet specifications.
Example harms considered include unfairness, misuse, and scalable oversight for autonomous systems.
The framework integrates ethics into the development process rather than just assessing systems after the fact.
Future work involves scaling the implementation across organizations and extending to more complex real-world systems.
The authors propose transparency, oversight, and accountability mechanisms to sustain trust.
The work combines moral philosophy, social science, and emerging AI techniques in an innovative way.
The value alignment approach could help address growing concerns about ethical AI systems.
Stakeholder participation highlighted additional harms like privacy violations.
Formal verification of abstract system models enabled "provable avoidance" of harms.
The framework demonstrates how ethics can be translated into concrete constraints and processes.
Overall, this provides a promising path toward developing AI that respects human values.
Next steps are studying transparent oversight mechanisms and scaling up collaboration.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: