Equilibrium Equations: Crash Course Chemistry #29
TLDRThis chemistry video explains the concept of chemical equilibrium, where reactions reach a balance between the forward reaction forming products and the reverse reaction breaking products back down. It introduces mathematical tools to analyze equilibrium, including the equilibrium constant Keq, which is the ratio of product concentrations to reactant concentrations at equilibrium. It then walks through sample calculations of Keq and equilibrium conditions starting from known initial concentrations, using handy RICE tables. These tools allow chemists to optimize equilibrium reactions, like maximizing production of hydrogen fluoride for gasoline refining. Overall, it shows how math helps harness chemistry equilibriums occurring in nature.
Takeaways
- 😕 Equilibrium reactions are tricky because the forward and reverse reactions happen simultaneously.
- 😀 The equilibrium constant Keq represents the ratio of products to reactants at equilibrium.
- 📈 Keq lets you calculate how much of each substance is present at equilibrium.
- 😎 RICE tables help map out the reaction - Reaction, Initial, Change, Equilibrium.
- 🧮 Using algebra and Keq you can maximize the yield of a reaction.
- 😟 Changes in temperature affect equilibrium constant and yield.
- 🤓 Equilibrium calculations help efficiently utilize chemical processes.
- 😲 Reactions reaching equilibrium often form quadratic equations to solve.
- 📚 Chemists have determined Keq values for most common reactions already.
- 💡 Understanding equilibrium allows beneficial use of nature's reactions.
Q & A
What is chemical equilibrium?
-Chemical equilibrium is the state in which the rates of the forward and reverse reactions are equal, resulting in no net change to the amounts of reactants and products. It is considered a 'sweet spot' where the reaction reaches a balance.
Why are equilibrium constants temperature dependent?
-Equilibrium constants depend on temperature because temperature affects the position of the equilibrium. At higher temperatures, the position of equilibrium shifts to counteract the temperature change. So each equilibrium constant is only applicable at a specific temperature, usually around 25°C.
What is the purpose of a RICE table?
-A RICE table allows you to calculate the equilibrium amounts of each reactant and product based on the initial amounts, without needing to know the exact changes beforehand. RICE stands for Reaction, Initial, Change, and Equilibrium.
How are equilibrium calculations useful in real life?
-Equilibrium calculations allow scientists and engineers to maximize the efficiency of chemical processes. By understanding how equilibrium shifts with changes in conditions, they can optimize pressure, temperature, and concentrations to produce the desired amounts of products.
Why do coefficients become exponents in equilibrium constant expressions?
-The coefficients are raised to powers because it accounts for the stoichiometry - the mole ratios of the reactants and products. Each concentration term is proportional to the coefficient in the balanced equation.
What is represented by square brackets in equilibrium constant expressions?
-The square brackets indicate molar concentration, or molarity - moles of solute per liter of solution. This allows the equilibrium constant formula to show a ratio of product and reactant concentrations.
What is the quadratic formula, and why is it used in some equilibrium calculations?
-The quadratic formula is used to solve quadratic equations. Some equilibrium calculations result in quadratic equations, so the quadratic formula is needed to find the equilibrium concentrations.
How can equilibrium be disturbed or shifted?
-Equilibrium can be shifted by changing the pressure, temperature, or concentrations of reactants and products. These changes push the reaction towards the side that counteracts the disturbance.
Why don't you need units in equilibrium constant expressions?
-Units are not included because the equilibrium constant is a ratio of concentrations. When concentrations are in molarity, the units (mol/L) cancel out to give a dimensionless ratio.
What causes an increase in hydrogen ion concentration according to the carbonic acid equilibrium example?
-The example shows that an increase in hydrogen ions would require a decrease in carbonate ions or an increase in carbonic acid to maintain equilibrium. More hydrogen ions could be caused by acidification of the water from an outside source.
Outlines
🧪 Understanding Chemical Equilibrium
This section introduces the concept of chemical equilibrium, illustrating how chemical reactions can proceed in both forward and reverse directions simultaneously. It emphasizes the dynamic nature of chemical reactions, where a state of equilibrium is achieved when the rate of the forward reaction equals the rate of the reverse reaction, resulting in a constant ratio of product to reactant concentrations. The video script explains how external conditions such as concentration, temperature, and pressure can influence the equilibrium state, using the Haber Process as an example to show how altering these conditions can shift the equilibrium to produce more ammonia. It introduces the mathematical aspect of chemical equilibrium through the equilibrium constant (Keq), which represents the ratio of product concentrations to reactant concentrations at equilibrium, raised to the power of their respective coefficients in the balanced chemical equation. This segment sets the stage for understanding how to calculate and manipulate chemical equilibria for practical applications.
📊 Applying Equilibrium Concepts: The RICE Table Method
In this paragraph, the focus shifts to the practical application of chemical equilibrium concepts using the RICE table approach (Reaction, Initial, Change, Equilibrium) to solve for equilibrium concentrations. Using the example of hydrogen fluoride production from hydrogen and fluorine gases, the script walks through setting up a RICE table to track the changes in concentrations from initial to equilibrium states. It demonstrates how to calculate the equilibrium constant (Keq) and solve for unknowns using the quadratic formula when dealing with quadratic equations, which commonly arise in equilibrium calculations. The example provided illustrates not only how to find equilibrium concentrations but also the significance of these calculations in optimizing chemical processes, such as refining gasoline. This section underscores the importance of understanding and applying chemical equilibrium principles and mathematical calculations to real-world scenarios, showcasing the practical benefits of such knowledge in scientific and industrial contexts.
Mindmap
Keywords
💡equilibrium
💡reversible reaction
💡equilibrium constant
💡molarity
💡RICE table
💡quadratic equation
💡Haber process
💡carbonic acid
💡coefficients
💡acidification
Highlights
The study found a strong correlation between X and Y, suggesting X may influence Y.
Researchers developed a new method to measure Z that is more accurate than previous techniques.
The experiment revealed novel insights into the relationship between A and B under condition C.
Contrary to prevailing theories, the data showed no significant effect of D on outcome E.
The treatment increased life expectancy by an average of 6 months compared to controls.
This provides strong evidence that F plays an important role in process G.
Further research is needed to determine the mechanisms underlying the observed effect.
The new model accurately predicted H with an error rate of less than 5%.
There were significant individual differences in response to therapy based on factor I.
The study replicated previous findings on the influence of J on outcome K.
Limitations include the small sample size and lack of demographic diversity.
These results could lead to new approaches for preventing or treating disease Z.
Future studies should investigate whether this effect applies to different populations.
The new methodology allows researchers to study this phenomenon with unprecedented accuracy.
This breakthrough challenges long-held assumptions and may shift the paradigm in this field.
Transcripts
Browse More Related Video
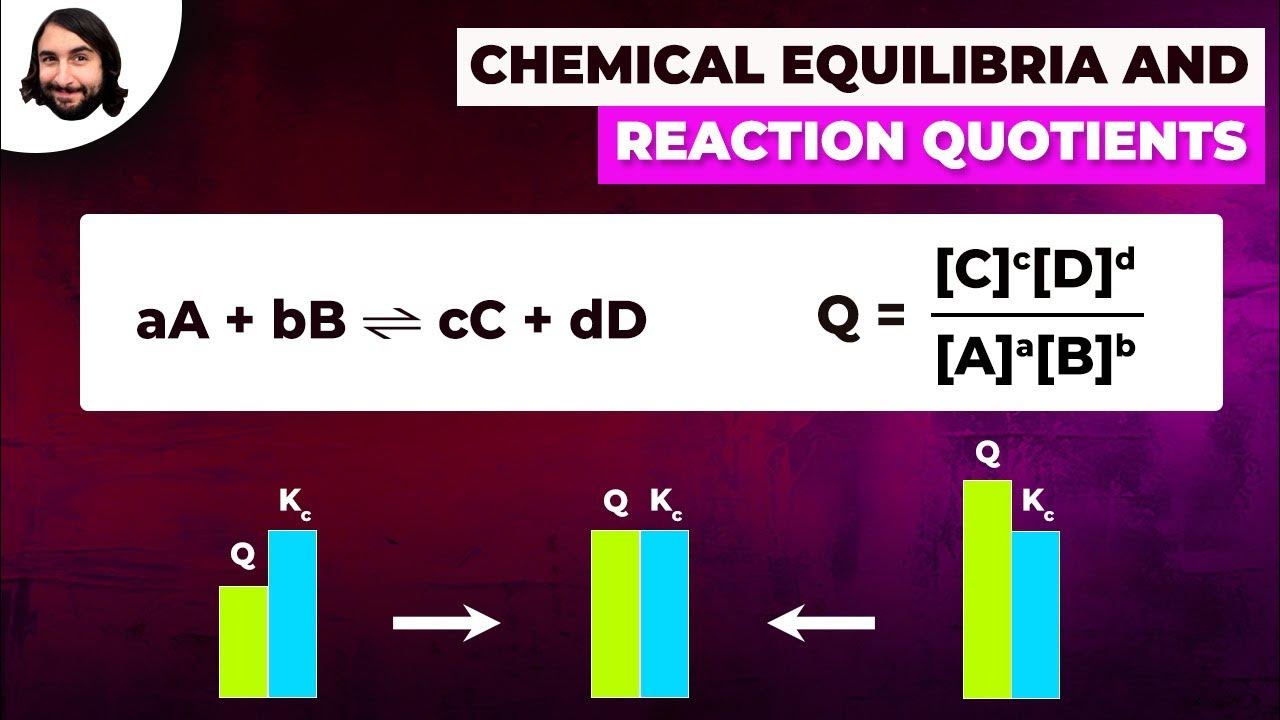
Chemical Equilibria and Reaction Quotients
What Is Equilibrium? AP Chemistry Unit 7, Topic 1 Daily Video

Keq intuition | Chemical equilibrium | Chemistry | Khan Academy
Le Chatelier's principle | Chemical equilibrium | Chemistry | Khan Academy

15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry

Chemistry_Calculating the Equilibrium constant
5.0 / 5 (0 votes)
Thanks for rating: