Predicting the Products of Chemical Reactions
TLDRThis educational video script focuses on predicting the products of chemical reactions, a skill crucial for students. It outlines the importance of identifying and classifying chemical reactions and understanding ion charges. The video covers three common types of reactions: single displacement, double displacement, and combustion. Single displacement involves metals replacing other metals in compounds or reacting with acids to displace hydrogen. Double displacement reactions include precipitation, where ions switch places to form a precipitate, and neutralization, where an acid and a base react to form a salt and water. Combustion reactions describe substances reacting with oxygen to produce heat, light, carbon dioxide, and water. The video emphasizes these concepts' significance in chemistry and their frequent appearance in exams, with additional resources available for further study.
Takeaways
- 🔍 Identifying and classifying types of chemical reactions is crucial for predicting products.
- 📚 Understanding how to determine the charges on ions is essential for balancing chemical equations.
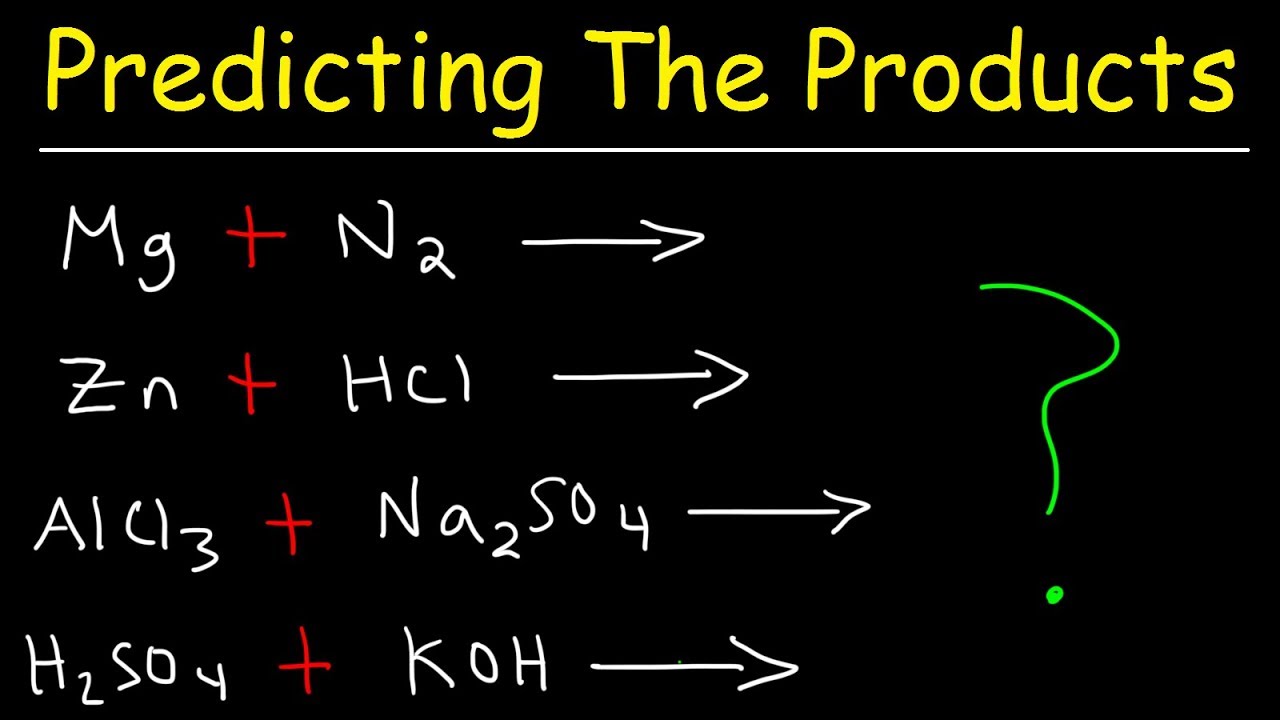
- 🧩 Single displacement reactions involve a metal replacing another in a compound, such as iron replacing copper in copper(II) sulfate.
- 🔋 Zinc can displace copper from copper(II) chloride, with the product being solid copper and aqueous zinc chloride.
- 🌟 In single displacement with acids, a metal displaces hydrogen, as in the reaction of zinc with hydrochloric acid producing zinc chloride and hydrogen gas.
- ⚖️ Balancing the charges of ions is necessary to predict the correct chemical formulas in reactions.
- 🤝 Double displacement reactions involve the exchange of ions between two compounds, leading to the formation of new products.
- 🏺 Precipitation reactions are a type of double displacement reaction where one product is an insoluble solid (precipitate).
- ⚗️ Neutralization reactions are double displacement reactions between an acid and a base, producing a salt and water.
- 🔥 Combustion reactions involve a substance reacting with oxygen to produce heat, light, carbon dioxide, and water.
- 📈 Practice is key for mastering chemical reactions, and additional resources like solubility tables and activity series can be helpful.
Q & A
What is the main goal of the video?
-The main goal of the video is to help students learn how to predict the products of chemical reactions when given just the reactants.
Why is it important to identify and classify the types of chemical reactions?
-Identifying and classifying chemical reactions is important because it helps in predicting the products of a reaction, which is a fundamental skill in chemistry.
What are the three most common types of reactions that the video focuses on?
-The three most common types of reactions focused on are single displacement, double displacement, and combustion reactions.
In a single displacement reaction involving metals, what happens to the metals involved?
-In a single displacement reaction involving metals, one metal replaces another in a compound, resulting in a new metal compound and the displaced metal as a separate entity.
What is a key factor to consider when predicting the products of a single displacement reaction involving metals?
-A key factor to consider is the charge on ions, as it is necessary to balance the charges to predict the correct products.
What is a double displacement reaction?
-A double displacement reaction is a type of reaction where the positive ions of two ionic compounds switch places, forming two new products, often resulting in a precipitate.
What are the two main types of double displacement reactions?
-The two main types of double displacement reactions are precipitation reactions and neutralization reactions.
What happens in a neutralization reaction?
-In a neutralization reaction, an acid and a base react to form a salt and water. The hydrogen ion (H⁺) from the acid donates a proton to the base, which provides a hydroxide ion (OH⁻), and they combine to form water.
What are the main products of a combustion reaction?
-The main products of a combustion reaction are carbon dioxide (CO₂) and water (H₂O), along with the release of heat and light.
Why is it important to know the diatomic gases in chemistry?
-Knowing the diatomic gases is important because they have specific forms (like Br2 for bromine, H2 for hydrogen, and O2 for oxygen) that are stable and commonly encountered in chemical reactions.
What additional resources does the video suggest for further help with chemical reactions?
-The video suggests a playlist with practice problems, solubility tables, activity series, and types of reactions for further help.
What is the significance of balancing the charges in predicting the products of a chemical reaction?
-Balancing the charges is significant because it ensures that the law of conservation of charge is maintained, which is a fundamental principle stating that charge cannot be created or destroyed in a chemical reaction.
Outlines
🧪 Understanding Chemical Reactions
This paragraph introduces the challenge of predicting chemical reaction products and emphasizes the importance of identifying and classifying reaction types and understanding ion charges. It outlines the video's focus on three common reaction types: single displacement, double displacement, and combustion. The paragraph also mentions a playlist for additional help on these topics.
🔍 Single Displacement Reactions
This section delves into single displacement reactions, explaining how a metal can replace another in a compound, as illustrated by the reaction between iron and copper(II) sulfate. It discusses the need to balance charges, especially when dealing with ions like zinc and chlorine. The paragraph provides an example problem for the viewer to practice predicting the products of such reactions and highlights the importance of knowing diatomic gases and their forms.
🤝 Double Displacement Reactions
Double displacement reactions are explored with a focus on two main types: precipitation and neutralization. Precipitation reactions involve the formation of an insoluble solid, or precipitate, when the positive ions of two ionic compounds switch places. An example given is the reaction between sodium nitrate and potassium iodide. Neutralization reactions, on the other hand, occur when an acid and a base react to form a salt and water. The example provided is the reaction between hydrochloric acid and sodium hydroxide, resulting in the formation of sodium chloride and water.
🔥 Combustion Reactions
The final paragraph discusses combustion reactions, where a substance reacts rapidly with oxygen, producing heat and light. The primary products of such reactions are carbon dioxide and water. Methane, the main component of natural gas, is used as an example to illustrate how carbon and hydrogen atoms combine with oxygen to form CO₂ and H₂O, respectively. The paragraph concludes by emphasizing the significance of understanding these three types of double displacement reactions for success in chemistry.
Mindmap
Keywords
💡Chemical Reactions
💡Reactants
💡Products
💡Classification of Reactions
💡Single Displacement Reactions
💡Double Displacement Reactions
💡Combustion Reactions
💡Ions and Charges
💡Diatomic Gases
💡Solubility
💡Neutralization
💡Activity Series
Highlights
Students can learn to predict the products of chemical reactions by identifying and classifying reaction types and understanding ion charges.
A link to a playlist is provided for further assistance on ion charges and reaction types.
Three common types of reactions to predict are single displacement, double displacement, and combustion.
In single displacement reactions, a metal can replace another in a compound, as seen with iron and copper.
Zinc can replace copper in an aqueous solution, forming zinc chloride and solid copper.
Metals can also displace hydrogen in acids, forming hydrogen gas and a metal chloride.
Double displacement reactions involve the exchange of ions between two compounds, often resulting in a precipitate.
Neutralization reactions occur when an acid and a base react to form a salt and water.
Combustion reactions involve a substance reacting with oxygen to produce heat, light, carbon dioxide, and water.
Methane combustion is an example of a rapid reaction with oxygen, producing CO₂ and H₂O.
The video emphasizes the importance of balancing charges in predicting reaction products.
Diatomic gases like bromine (Br2), hydrogen (H2), and oxygen (O2) are highlighted for their consistent bonding.
The video provides an example of a single displacement reaction involving zinc and hydrochloric acid.
Magnesium chloride is formed when magnesium displaces hydrogen from hydrochloric acid, with charge balance in mind.
The video does not necessarily present balanced chemical equations for simplicity.
Further assistance and practice problems are available in a linked playlist for those needing extra help.
Combination and decomposition reactions will be covered in a subsequent video.
Understanding these reaction types is crucial for success in chemistry and often tested on exams.
Transcripts
Browse More Related Video

5 Types of Chemical Reactions (Chemistry) + Activity Series, Solubility Rules

Chemistry Lesson: Types of Chemical Reactions
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems

Types of Chemical Reactions

Chemical Reactions

Chemical Reactions (3 of 11) Combustion Reactions, An Explanation
5.0 / 5 (0 votes)
Thanks for rating: