The Ideal Gas Law: Crash Course Chemistry #12
TLDRThis video explores gases and the mathematical relationships describing their behavior. It traces the history of formulating the Ideal Gas Law, from Boyle's early 17th century linking of pressure and volume, to the later contributions of Charles and Avogadro involving temperature and moles of gas. Though named for Robert Boyle, the law's origins owed more to lesser-known researchers like Henry Power. The summary explains how the variables of pressure, volume, temperature, and quantity in the Ideal Gas Law interrelate at the particle level. It notes that while the law accurately describes gaseous behavior under many conditions, gases do not always behave ideally, deviating at high pressures and low temperatures.
Takeaways
- 😀 Boyle's Law links pressure and volume of gases, but was likely discovered by Towneley and Power, not Boyle
- 🌟 The Ideal Gas Law links pressure, volume, temperature, and number of moles of gas
- 🔬 Understanding movement of gas molecules explains pressure, volume, and temperature changes
- 📈 Increasing volume decreases pressure if temperature and gas amount stays constant
- 😎 Increasing temperature increases pressure as molecules move faster
- 🧪 Chemists like Charles, Avogadro and Boyle derived equations linking gas features
- 🏺 The Ideal Gas Law unifies these equations in one simple relationship
- ⚗️ The gas constant R and moles n relate to kinetic energy of molecules
- 🎉 Using the Ideal Gas Law, if you know 3 features you can calculate the 4th
- i STP and absolute zero are handy reference points for gas calculations
Q & A
Who first established the mathematical relationship between pressure and volume in gases?
-The first mathematical description of the relationship between pressure and volume in gases is attributed to Richard Towneley, a wealthy Englishman, who discussed his experiments with Robert Boyle. However, it was named Boyle's Law after Boyle published a paper about it.
What contribution did Henry Power make regarding the discovery of Boyle's Law?
-Henry Power, a working class scientist and Towneley's family friend and physician, conducted many of the experiments that led to the discovery of the relationship between pressure and volume in gases. However, he did not receive credit at the time as Boyle published the findings first after discussing them with Towneley.
What is the equation for the Ideal Gas Law, and what do the variables represent?
-The Ideal Gas Law equation is: PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the universal gas constant, and T is temperature in Kelvin.
Why does crushing a heated soda can in ice water demonstrate the Ideal Gas Law?
-Crushing a heated soda can in ice water causes dramatic drops in temperature, number of moles, pressure, and volume inside the can. This demonstrates the Ideal Gas Law because the changes in these variables are interrelated, just as the law describes.
What does the universal gas constant represent and what is its value?
-The universal gas constant (R) is 8.3145 liters kilopascals per kelvin mole. It is a proportionality constant in the Ideal Gas Law equation.
What does standard temperature and pressure (STP) mean?
-Standard temperature and pressure (STP) refers to a standard set of conditions used as a reference point. In chemistry, STP is defined as 0 degrees Celsius (273.15 K) and 100 kilopascals (100,000 pascals) of pressure.
Why does the volume occupied by one mole of an ideal gas differ under different conditions?
-Under STP (standard temperature and pressure) conditions, one mole of an ideal gas occupies 22.4 liters. However, the volume depends on temperature and pressure as described by the Ideal Gas Law. So under different conditions, one mole occupies a different volume.
What causes the pressure inside a balloon to increase when you decrease its volume?
-Decreasing the volume means the gas molecules inside have less space to move around. They hit the sides of the balloon more often, exerting more pressure.
How did the work of different scientists like Boyle, Charles and Avogadro contribute to the Ideal Gas Law equation?
-Each scientist derived different gas laws experimentally, relating pressure, volume, temperature and number of moles. Their work described different forms of the same underlying relationship, which was eventually combined into the single Ideal Gas Law equation.
Why do real gases deviate from the Ideal Gas Law under certain conditions?
-The Ideal Gas Law assumes gases consist of particles with no volume that do not attract or repel each other. Under conditions like low temperatures or high pressures, these assumptions break down as intermolecular forces become more significant, causing deviations.
Outlines
😀 What gases are and Boyle's Law relating pressure and volume
This paragraph introduces gases, which are all around us, and describes how waving your hands allows you to feel gases. It then presents Boyle's Law, which relates pressure and volume - as volume decreases in a closed system, pressure increases. This law is attributed to the wrong person - it should actually be named after Towneley and Power.
😲 The Ideal Gas Law relating pressure, volume, temperature, and moles
This paragraph explains the Ideal Gas Law, which relates pressure, volume, temperature, and number of moles of gas. It shows how this law connects the discoveries of Boyle, Charles, and Avogadro. It also defines each variable - pressure, volume, moles, the gas constant R, and temperature. It then demonstrates the law by crushing a heated soda can in cold water.
Mindmap
Keywords
💡Boyle's Law
💡ideal gas
💡pressure
💡temperature
💡volume
💡moles
💡atmosphere
💡absolute zero
💡standard temperature and pressure (STP)
💡Universal Gas Constant (R)
Highlights
Robert Boyle was a super rich Englishman, raised in Ireland. His father was so rich that he paid another family to raise his children.
Power was working on a publication that would have snared him the position as discover of, the relationship between the pressure and volume of a gas. But Boyle published first, attributing Towneley as the sole researcher, ensuring that Power's contributions were all but lost to history.
For a given amount of gas at a constant temperature, pressure times volume always equals the same number. But where is that constant coming from, and why is it different for different amounts of gas at different temperatures?
Charles discovered that volume divided by temperature equals a constant, as long as the pressure remains the same. And then Avogadro figured out that volume divided by the number of moles in the container, at a constant pressure and temperature gave yet another constant.
Pressure times volume is equal to the number of moles of substances times a constant times temperature. P V equals n R T: The Ideal Gas Law, which works for all gases as long as they behave themselves.
In that same way the atoms and molecules that make up gases, are bouncing against things, applying pressure to them. This balloon is inflated because the molecules are bouncing around inside of it, bumping into the inside of the balloon harder than the molecules bouncing off the outside of the balloon.
Completely by chance, one atmosphere is equal to 101325 pascals, but that's so close that we often just say that one atmosphere is 100,000 pascals or 100 kilopascals.
Temperature, is experienced by you and me as hot or cold but at the atomic level it's kinetic energy. Literally, how fast or slow the average particle is moving. So if temperature goes up, so will the pressure as the particles are moving faster, and thus will run into the sides of the container more often.
Now I understand that you probably don't think this is as cool as I do, but understanding the physical reality of atoms and molecules smacking into things is, a special kind of beautiful for me.
It's also pretty cool that if you know any 3 things about a gas, you can figure out the fourth using the ideal gas law.
Absolute zero is the temperature at which all movement of all particles stops. It is zero kelvins or -273.15 degrees Celsius.
how none of those people were Robert Boyle, and how the Ideal Gas Equation allows you to find out pressure, volume, temperature or number of moles, as long as you know three of those four things.
One mole of any ideal gas takes up 22.4 liters of space at STP, which is a fact that can simplify a lot of calculations.
STP means standard temperature and pressure, which according to the lords of chemistry is 0 degrees Celsius and 100,000 pascals or 100 kilopascals.
Transcripts
Browse More Related Video
Ideal Gas Problems: Crash Course Chemistry #13
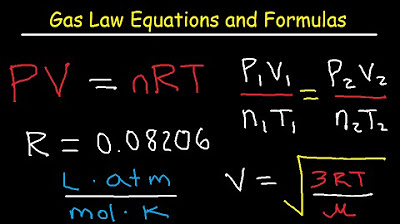
Gas Laws - Equations and Formulas

Ideal Gas Law

Kinetic Molecular Theory and the Ideal Gas Laws

Introduction to partial pressure | Gases and kinetic molecular theory | Chemistry | Khan Academy

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
5.0 / 5 (0 votes)
Thanks for rating: