Partial Pressures & Vapor Pressure: Crash Course Chemistry #15
TLDRThis video explores gases and gas laws, building on foundational theories of British chemist John Dalton. After explaining concepts like partial pressure and mole fraction, it delves into combining gases and observing the total pressure they exert. It outlines an experiment mixing baking soda and vinegar to produce carbon dioxide, then collecting and measuring the gas. Calculations using variables like vapor pressure and volume quantify the amount of carbon dioxide captured. Through this accessible experiment, the video illustrates gas behavior and collection methods, spurring curiosity about chemistry.
Takeaways
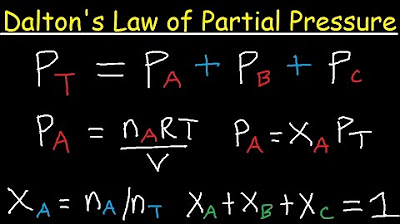
- 😀 Dalton's research into combining gases led to the Law of Partial Pressures, which states that the total pressure of a gas mixture equals the sum of the partial pressures of each individual gas.
- 😮💨 The ratio of the moles of an individual gas to the total moles in a mixture is called the mole fraction. This is related to partial pressures through the Ideal Gas Law.
- 🚰 When collecting a gas over water, the vapor pressure of water mixes with and adds to the pressure of the collected gas. This must be accounted for in calculations.
- 😑 Vinegar (acetic acid) and baking soda (sodium bicarbonate) react to form sodium acetate, carbon dioxide, and water. The rapid production of CO2 gas is what causes the dramatic bubbling.
- 🧪 Dalton used scientific measurement and reasoning to develop an early atomic theory and describe how atoms combine in whole-number ratios.
- 😶🌫️ Gases exert pressure by having their particles move around and bump into container walls. Faster movement and more particles lead to higher pressure.
- 🥽 Scuba tanks contain oxygen and helium. Using the Ideal Gas Law, the partial pressures of each gas can be calculated from their amounts, then added to get the total tank pressure.
- 🎈 The pressure, temperature, volume, and moles of a gas are connected mathematically through the Ideal Gas Law equation.
- ☝ The total pressure exerted by a mixture of non-reacting gases equals the sum of the pressures each gas would exert if it were alone.
- 📝 Proust's Law of Definite Proportions, stating elements combine in fixed ratios, was expanded by Dalton into the Law of Multiple Proportions.
Q & A
What gas law did John Dalton develop from his research on gases?
-Dalton developed the Law of Partial Pressures, which states that the total pressure exerted by a mixture of gases is equal to the sum of the pressures that the individual gases would exert if they were alone.
What is the mole fraction of a gas in a mixture?
-The mole fraction of a gas is the ratio of the number of moles of that gas to the total number of moles of all gases in the mixture.
Why does water have a vapor pressure?
-Liquid water constantly gives off water vapor molecules that have enough kinetic energy to escape the liquid. The pressure exerted by these vapor molecules is called the vapor pressure.
What happens when baking soda and vinegar react?
-Baking soda (sodium bicarbonate) reacts with vinegar (acetic acid) to produce sodium acetate, carbon dioxide, and water. The rapid production of carbon dioxide gas leads to the dramatic bubbling.
How can you collect a gas produced from a reaction over water?
-By bubbling the gas through a column of water, the gas gets trapped and can be collected. However, the total pressure includes the vapor pressure of the water, so this must be subtracted to determine the actual pressure of the collected gas.
What information is needed to calculate the moles of gas collected using the method described?
-You need the volume of gas collected, the total pressure, the vapor pressure of water at the temperature used (to subtract), the temperature, and the gas constant R.
What was Proust's Law of Definite Proportions?
-Proust found that elements always combine in fixed mass ratios in compounds. Dalton expanded this to develop the Law of Multiple Proportions about elemental ratios.
Why is helium often mixed with oxygen in scuba diving tanks?
-Adding helium helps prevent decompression sickness since helium comes out of solution in the blood faster than nitrogen upon surfacing.
What analogy did the narrator use to explain gas behavior?
-The narrator compared gases mixing and exerting pressure to groups of dignified adults and chaotic children bumping into walls at a party.
What are two inaccuracies in Dalton's atomic theory?
-Dalton thought all atoms of an element were identical and that compounds were formed by atoms simply sticking together. We now know atoms have isotopes and compounds involve chemical bonds.
Outlines
😀 Analogies and Dalton's Atomic Theory
Paragraph 1 introduces John Dalton's atomic theory through analogies of a formal state dinner attended by 300 adults versus a birthday party attended by 300 energetic 5-year-olds. It sets up how these scenarios relate to Dalton's studies of mixing gases and discovering the law of partial pressures.
😊 Dalton's Law of Partial Pressures
Paragraph 2 explains Dalton's law of partial pressures in more detail - that the total pressure exerted by a gas mixture equals the sums of the pressures each gas would exert individually. It shows examples applying this law, like calculating the pressure in a scuba tank and finding the partial pressure of oxygen in air.
🧪 Collecting and Measuring Gases
Paragraph 3 demonstrates an experiment to collect carbon dioxide gas produced from a chemical reaction. It explains the concept of vapor pressure, shows how to collect a gas over water, and calculates the amount of carbon dioxide gas collected using the ideal gas law.
Mindmap
Keywords
💡Law of Partial Pressures
💡mole fraction
💡vapor pressure
💡chemical reaction
💡foam
💡gas collection
💡graduated cylinder
💡Ideal Gas Law
💡atmospheric pressure
💡molar mass
Highlights
Dalton expanded Proust's Law of Definite Proportions to develop the Law of Multiple Proportions, leading to the gas law of partial pressures
The total pressure exerted by a gas mixture equals the sum of the pressures exerted by each individual gas
The ratio of individual gas moles to total moles in a mixture is called the mole fraction
Mole fractions and partial pressures are related through the ideal gas law
Water vapor mixes with collected gases, so vapor pressure must be subtracted from total pressure
Baking soda and vinegar produce carbon dioxide gas through a chemical reaction
Capturing gas in a water column allows measurement of gas quantity and pressure
Measurements allow calculation of moles of captured carbon dioxide gas
Subtract water vapor pressure from total pressure to get carbon dioxide pressure
Use measurements in the ideal gas law to calculate moles of captured carbon dioxide
Dalton's work enabled both atomic theory and gas behavior laws
Gases combine additively, allowing calculation of total and partial pressures
Chemical reaction of vinegar and baking soda explained
Technique to collect gas over water demonstrated
Amount of captured carbon dioxide gas successfully measured
Transcripts
Browse More Related Video
Dalton's Law of Partial Pressure Problems & Examples - Chemistry

Chemistry | Gas Explosion Experiment at Home! | Acid and Base Reaction | Science Experiment

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion

Henry's Law Explained - Gas Solubility & Partial Pressure - Chemistry Problems

Introduction to partial pressure | Gases and kinetic molecular theory | Chemistry | Khan Academy

9.3 Additional Gas Laws | Dalton's Law and Graham's Law | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: