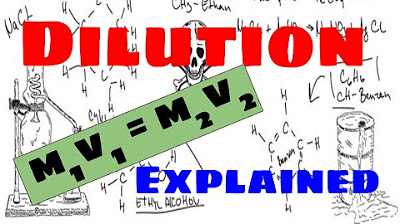
The C1V1 = C2V2 Equation Explained
TLDRThe video script provides a clear explanation of the c1v1=c2v2 equation, which is fundamental in chemistry for calculating concentrations and volumes in solutions. It breaks down the equation into its components: c1 (starting concentration), v1 (starting volume), c2 (final concentration), and v2 (final volume), emphasizing that the product of the starting concentration and volume is equal to that of the final concentration and volume. The script then demonstrates how to rearrange the equation to solve for an unknown variable when three of the four are known. Two examples are given: calculating the volume of a primer solution needed for a PCR reaction to achieve a specific final concentration, and determining the amount of water to add to pure ethanol to reach a 70% ethanol solution. The step-by-step process for each calculation is outlined, making the concept accessible to viewers.
Takeaways
- 🧪 The c1v1 = c2v2 equation is a fundamental concept in chemistry, representing the relationship between initial and final concentrations and volumes in a solution.
- 🔍 c1 represents the starting concentration, v1 the starting volume, c2 the final concentration, and v2 the final volume of a solution.
- 📐 If you know three of the four variables (c1, v1, c2, v2), you can solve for the fourth using the c1v1 = c2v2 equation.
- ✂️ To find an unknown variable, rearrange the equation to isolate that variable, then perform the necessary calculations.
- 📉 For calculating the starting concentration when the starting volume, final concentration, and final volume are known, divide the final concentration by the starting volume and multiply by the final volume.
- 📈 To determine the final volume when given the initial volume and concentrations, move c2 to the other side of the equation and solve accordingly.
- 🌡️ An example given in the script involves calculating the volume of a 10 micromolar forward primer solution needed for a PCR reaction with a final concentration of 0.4 micromolar in a 25 microliter total reaction volume.
- 🧬 The solution to the PCR example is 1 microliter, found by dividing the final volume by the starting concentration and multiplying by the final concentration.
- 💧 Another example demonstrates calculating the amount of water needed to add to 100 milliliters of 100% ethanol to achieve a 70% ethanol solution.
- 📝 The calculation for the ethanol example results in a final volume of 142.9 milliliters, meaning 42.9 milliliters of water must be added to the ethanol.
- 🔑 The c1v1 = c2v2 equation is versatile and can be applied to a wide range of chemistry problems involving dilutions and concentration adjustments.
- 🎓 Understanding and applying this equation is crucial for students and professionals in fields such as biology, chemistry, and medicine where precise solution concentrations are often required.
Q & A
What does the equation C1V1 = C2V2 represent?
-The equation C1V1 = C2V2 represents the principle that the product of the initial concentration (C1) and initial volume (V1) of a solution is equal to the product of the final concentration (C2) and final volume (V2).
What does C1 in the equation C1V1 = C2V2 stand for?
-In the equation, C1 stands for the starting concentration of the solution.
What does V1 represent in the context of the C1V1 = C2V2 equation?
-V1 represents the starting volume of the solution in the C1V1 = C2V2 equation.
How is C2 defined in the provided script?
-C2 is defined as the final concentration of the solution in the script.
What does V2 signify in the C1V1 = C2V2 equation?
-V2 signifies the final volume of the solution in the C1V1 = C2V2 equation.
If you know three of the four variables in the C1V1 = C2V2 equation, what can you determine?
-If you know three of the four variables (C1, V1, C2, V2), you can determine the fourth unknown variable using the equation.
How do you rearrange the equation to solve for the starting concentration?
-To solve for the starting concentration (C1), you rearrange the equation to C1 = (C2 * V2) / V1.
What is the example given in the script to demonstrate the use of the C1V1 = C2V2 equation?
-The example given is calculating the amount of a 10 micromolar forward primer solution to add to a PCR reaction with a total reaction volume of 25 microliters to achieve a final concentration of 0.4 micromolar.
How much microliter of the 10 micromolar forward primer solution is needed to achieve the final concentration in the PCR example?
-1 microliter of the 10 micromolar forward primer solution is needed to achieve the final concentration in the PCR example.
What is the second example provided in the script?
-The second example is calculating the amount of water needed to add to 100 milliliters of pure ethanol to make a final concentration of 70% ethanol solution.
How much water in milliliters is needed to be added to 100 milliliters of pure ethanol to achieve a 70% ethanol solution?
-42.9 milliliters of water need to be added to 100 milliliters of pure ethanol to achieve a 70% ethanol solution.
What is the significance of the C1V1 = C2V2 equation in chemistry?
-The C1V1 = C2V2 equation is significant in chemistry as it allows for the calculation of unknown volumes or concentrations in dilutions or mixing of solutions, which is crucial in many laboratory procedures and chemical analyses.
Outlines
🧪 Understanding the C1V1 = C2V2 Equation
This paragraph explains the concept of the C1V1 = C2V2 equation, which is a fundamental principle in chemistry for calculating concentrations and volumes. It describes that C1 represents the initial concentration, V1 the initial volume, C2 the final concentration, and V2 the final volume. The equation states that the product of the initial concentration and volume is equal to the product of the final concentration and volume. This can be rearranged to solve for any one of the variables when the other three are known. An example is provided where the starting concentration of a substance is calculated given the starting volume, final concentration, and final volume. The calculation involves dividing the final concentration by the initial concentration and then multiplying by the final volume to find the initial volume.
📏 Applying the C1V1 = C2V2 Equation to Real-World Scenarios
The second paragraph demonstrates the application of the C1V1 = C2V2 equation in two practical examples. The first example involves calculating the volume of a 10 micromolar forward primer solution needed for a PCR reaction with a total reaction volume of 25 microliters to achieve a final concentration of 0.4 micromolar. By rearranging the equation and substituting the known values, it is determined that 1 microliter of the primer solution is required. The second example shows how to calculate the amount of water needed to be added to 100 milliliters of pure ethanol to achieve a final concentration of 70% ethanol solution. The calculation leads to a final volume of 142.9 milliliters, indicating that 42.9 milliliters of water must be added to the ethanol to reach the desired concentration. This paragraph concludes with a summary of the C1V1 = C2V2 equation's utility.
Mindmap
Keywords
💡C1V1=C2V2 equation
💡Starting concentration (C1)
💡Starting volume (V1)
💡Final concentration (C2)
💡Final volume (V2)
💡Rearranging the equation
💡PCR reaction
💡Micromolar
💡Total reaction volume
💡Dilution
💡Ethanol solution
💡Percentage concentration
Highlights
The c1v1 equals c2v2 equation is explained in the video, providing a fundamental concept in concentration and volume calculations.
c1 represents the starting concentration, v1 the starting volume, c2 the final concentration, and v2 the final volume in the equation.
The equation implies that the product of the starting concentration and volume is equal to the product of the final concentration and volume.
Given three out of four variables, one can solve for the unknown using the c1v1=c2v2 equation.
A practical example involves calculating the starting concentration when the final concentration, final volume, and starting volume are known.
The equation can be rearranged to solve for the unknown variable, such as starting concentration or final volume.
A specific example given in the video is calculating the amount of 10 micromolar forward primer solution for a PCR reaction.
The total reaction volume in the example is 25 microliters, aiming for a final concentration of 0.4 micromolar.
By applying the c1v1=c2v2 equation, it is determined that 1 microliter of the primer solution is needed.
Another example demonstrates how to calculate the amount of water needed to create a 70% ethanol solution using 100 milliliters of 100% ethanol.
The c1v1=c2v2 equation is used to calculate the final volume of the ethanol solution, which turns out to be 142.9 milliliters.
Subtracting the initial 100 milliliters of ethanol from the final volume gives the amount of water needed, which is 42.9 milliliters.
The video provides a clear and detailed explanation of how to use the c1v1=c2v2 equation in practical scenarios.
The method can be applied to various solutions and concentrations, making it a versatile tool in scientific calculations.
The video's examples are well-chosen to illustrate the application of the equation in real-world situations.
The c1v1=c2v2 equation is a fundamental concept that is essential for understanding concentration and volume relationships.
The video's approach to explaining the equation is engaging and informative, making complex concepts accessible.
The practical applications of the c1v1=c2v2 equation highlighted in the video are beneficial for students and professionals in scientific fields.
The video's examples serve as a practical guide for anyone needing to perform concentration and volume calculations.
The c1v1=c2v2 equation is a key tool for solving various problems in chemistry and biology.
The video's content is valuable for anyone looking to understand or apply concentration and volume calculations.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: