Introduction to the atom | Chemistry of life | Biology | Khan Academy
TLDRThe video script delves into the philosophical and scientific aspects of atoms, exploring their structure with protons, neutrons, and electrons. It challenges the traditional model of electron orbits, introducing the concept of quantum mechanics and probability functions. The script also discusses isotopes and atomic weights, emphasizing that most of an atom's volume is actually empty space, questioning our understanding of physical reality.
Takeaways
- 🍎 The concept of atoms has philosophical roots, dating back to ancient philosophers who pondered the indivisible building blocks of matter.
- 🤔 The term 'atom' comes from the Greek word for 'uncuttable' or 'indivisible', but modern science has shown that atoms can be divided into subatomic particles like neutrons, protons, and electrons.
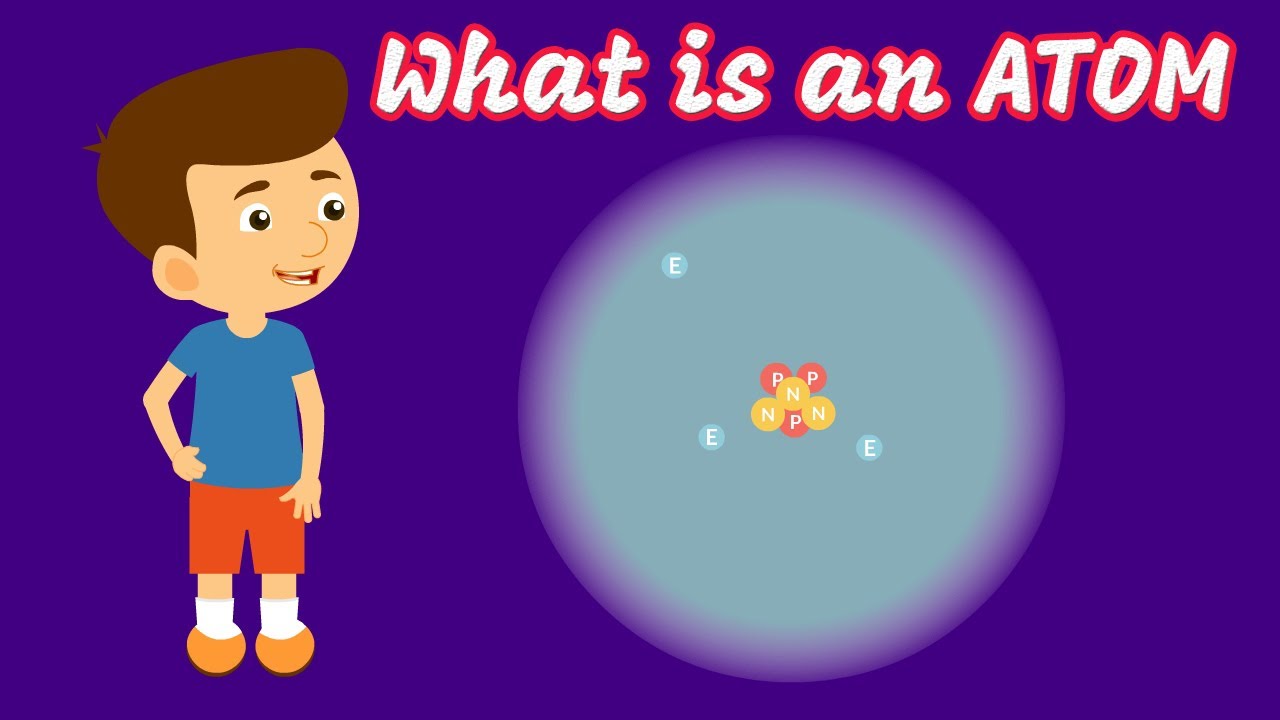
- 🔬 An atom consists of a nucleus containing neutrons and protons, with electrons orbiting around it in a cloud-like formation rather than fixed paths.
- ⚛️ The number of protons in an atom defines the element, and this is referred to as the atomic number.
- 📊 The atomic weight of an element is an average that takes into account the different isotopes and their abundance in nature.
- 🌐 Isotopes are versions of the same element with a different number of neutrons, leading to variations in the atomic mass.
- 💫 Electrons exist as a probability distribution around the nucleus, described by an orbital, rather than fixed orbits.
- 🚀 The nucleus of an atom is incredibly small compared to the overall space occupied by the electron cloud, often representing only a tiny fraction of the atom's volume.
- ✨ Most of the space within an atom is actually empty, challenging our everyday perception of solid matter.
- 🌟 The behavior of subatomic particles blurs the line between physical reality and information, as their existence is often described in terms of probabilities and mathematical functions.
- 🌌 Quantum mechanics further complicates the understanding of atoms, showing that traditional notions of particles and waves do not fully apply at such small scales.
Q & A
What is the philosophically interesting aspect of atoms mentioned in the script?
-The philosophically interesting aspect of atoms is that they blur the line between physical reality and information, questioning the nature of matter and particles as defined in everyday life.
What is the historical perspective on the concept of atoms?
-Historically, philosophers like Democritus and Leucippus proposed the idea of atoms as uncuttable or indivisible units of matter. They thought that if you could keep dividing matter, you would eventually reach a point where it could not be divided further.
What are the three fundamental particles that make up an atom?
-An atom is made up of three fundamental particles: neutrons, protons, and electrons.
How does the script describe the traditional model of electrons in an atom?
-Traditionally, electrons were thought to orbit the nucleus in fixed paths, similar to planets orbiting the Sun. However, this model has been shown to be incorrect, and electrons are now understood to exist in a probability cloud or orbital around the nucleus.
What is an orbital in the context of quantum mechanics?
-An orbital is a mathematical probability function that describes the likelihood of finding an electron in a particular region around the nucleus of an atom. It replaces the older model of fixed electron orbits.
How is an element identified?
-An element is identified by the number of protons in its nucleus, which is known as the atomic number.
What is the significance of the atomic weight of an element?
-The atomic weight of an element is the average mass of all the atoms of that element, taking into account the different isotopes and their relative abundances. It is a weighted average that reflects the natural distribution of isotopes.
What is an isotope?
-An isotope is a variant of an element that has the same number of protons but a different number of neutrons in its nucleus. Isotopes of an element have different atomic masses but share the same chemical properties.
How does the script describe the mass distribution within an atom?
-The script describes the mass of an atom as being primarily concentrated in the nucleus, with the electrons contributing very little to the overall mass. The nucleus contains the protons and neutrons, each with a mass approximately equal to one atomic mass unit.
What philosophical implications does the structure of atoms have?
-The structure of atoms raises philosophical questions about the nature of reality and matter. If most of an atom's volume is empty space, it challenges our intuitive understanding of solidity and the physical world.
How does the script illustrate the scale of atoms?
-The script uses the example of a helium atom, explaining that its nucleus is one femtometer in size, and one angstrom is equivalent to 100,000 femtometers. This helps to illustrate the incredibly small scale at which atoms operate.
Outlines
🌟 Introduction to Atoms and Philosophical Significance
This paragraph introduces the concept of atoms as the fundamental building blocks of matter, highlighting their philosophical importance from the very beginning of chemistry. It discusses the historical perspective of philosophers who conceptualized atoms as indivisible units, akin to cutting an apple into smaller and smaller pieces until nothing more can be divided. The modern understanding of atoms as composed of protons, neutrons, and electrons is contrasted with this early philosophical construct, emphasizing the evolution of atomic theory and its abstract nature. The paragraph also touches on the philosophical implications of atoms, questioning the nature of physical reality and the true meaning of matter at the atomic scale.
🔬 Atomic Structure and Quantum Mechanics
The paragraph delves into the structure of atoms, describing the nucleus composed of protons and neutrons, and electrons orbiting around it. It challenges the traditional model of electrons orbiting the nucleus like planets around the Sun, instead introducing the concept of electrons as probability distributions, or orbitals, which represent the likelihood of finding an electron in a particular location around the nucleus. This discussion sets the stage for a deeper exploration of quantum mechanics, emphasizing the counterintuitive and complex behavior of particles at the atomic level, and how this challenges our everyday understanding of matter and particles.
📊 Identifying Elements and the Role of Protons
This section focuses on the identification of elements based on the number of protons in an atom, known as the atomic number. It explains that the atomic number is what fundamentally defines an element, with the periodic table of elements organized accordingly. The paragraph also addresses the concept of charge, with electrons carrying a negative charge, protons a positive charge, and neutrons being neutral. The importance of protons in determining the identity of an element is emphasized, and the notion of isotopes is introduced, explaining that isotopes are variants of an element with different numbers of neutrons but the same number of protons.
🌐 Atomic Weight and the Concept of Isotopes
The paragraph discusses the concept of atomic weight, which is the average mass of an element's atoms, taking into account the different isotopes and their relative abundances. It clarifies that the atomic weight is not the simple sum of protons and neutrons due to the existence of isotopes. The example of carbon is used to illustrate this point, explaining how the atomic weight of carbon (approximately 12.0107) reflects the average mass of all carbon atoms found on Earth, including the more common carbon-12 and the rarer carbon-14 isotopes. The paragraph reinforces the idea that isotopes are different versions of the same element, differing only in their neutron count.
🌌 The Scale and Implications of Atomic Structure
The final paragraph of the script explores the scale of atoms and their components, emphasizing the vast empty space within atoms. It describes the nucleus as an infinitesimally small fraction of the atom's volume, with electrons occupying an even smaller proportion. The idea that most of the space we perceive as solid matter is actually empty is highlighted, challenging our perception of reality and solidity. The paragraph concludes by reflecting on the philosophical implications of this atomic perspective, leaving the audience with a profound appreciation for the vast, empty spaces within the seemingly solid objects that make up our world.
Mindmap
Keywords
💡Atom
💡Philosophically interesting
💡Neutron
💡Proton
💡Electron
💡Quantum mechanics
💡Orbital
💡Atomic number
💡Periodic table of elements
💡Isotope
💡Atomic mass unit (amu)
💡Atomic weight
Highlights
Chemistry begins with philosophically interesting concepts, such as atoms, right from the start.
The concept of the atom dates back to ancient philosophers who pondered the idea of an indivisible particle.
The word 'atom' comes from the Greek for 'uncuttable' or 'indivisible', though we now know atoms can be divided into more fundamental particles.
An atom consists of a nucleus containing neutrons and protons, with electrons orbiting around it.
The traditional model of electrons orbiting the nucleus is akin to planets orbiting the Sun, but this is an oversimplification.
Electrons are better described by a probability distribution rather than a fixed orbit, a concept known as an 'orbital'.
The behavior of electrons at the atomic level blurs the line between physical reality and information, questioning what matter truly is.
An element is defined by the number of protons it has, known as the atomic number.
The atomic weight of an element is the average mass of all the isotopes of that element found on Earth.
Isotopes are versions of the same element with different numbers of neutrons.
Most of an atom's volume is actually empty space, with the nucleus containing most of the atom's mass.
The nucleus of an atom constitutes only a tiny fraction of the atom's total volume, with electrons occupying a large, yet mostly empty, space around it.
The concept of atoms challenges our understanding of solidity and physical reality, as most of what we perceive as solid is actually empty space at the atomic level.
The philosophical implications of atomic theory question the nature of reality and the true meaning of matter.
The study of atoms and their properties is fundamental to understanding the universe and the nature of physical reality.
Quantum mechanics further complicates and enriches our understanding of atoms, electrons, and the behavior of matter at the smallest scales.
The realization that most of an atom is empty space has profound implications for our understanding of the composition of everyday objects.
Transcripts
Browse More Related Video

Structure of the Atom - Proton, Neutron, Electron - Atomic Number & Mass Number - [1-2-6]
What is an Atom? - Structure of an Atom - Atom video for kids

Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy

The Quantum Mechanical model of an atom. What do atoms look like? Why?

Basic Atomic Structure: A Look Inside the Atom
Applied Chemistry_Atomic Charecteristics_ Lecture 06 for Polytechnic 1st Semester
5.0 / 5 (0 votes)
Thanks for rating: