Structure of the Atom - Proton, Neutron, Electron - Atomic Number & Mass Number - [1-2-6]
TLDRThis educational lesson delves into modern atomic structure, shifting from historical discoveries to present understandings within the past century. It explores the atom's components—protons, neutrons, and electrons—and their behavior, challenging traditional models with quantum mechanics insights. The lesson revisits the notion of electrons as not merely particles but entities with wave-like properties, contributing to our quantum view of matter. Further, it introduces atomic mass units, the concept of isotopes, and their significance in chemistry, emphasizing the atom's nearly empty space and the nucleus's mass concentration. Through examples like the helium atom and carbon isotopes, it elucidates atomic number, mass number, and the stability of atomic structures, offering a foundational grasp of atoms' complex nature for further chemical studies.
Takeaways
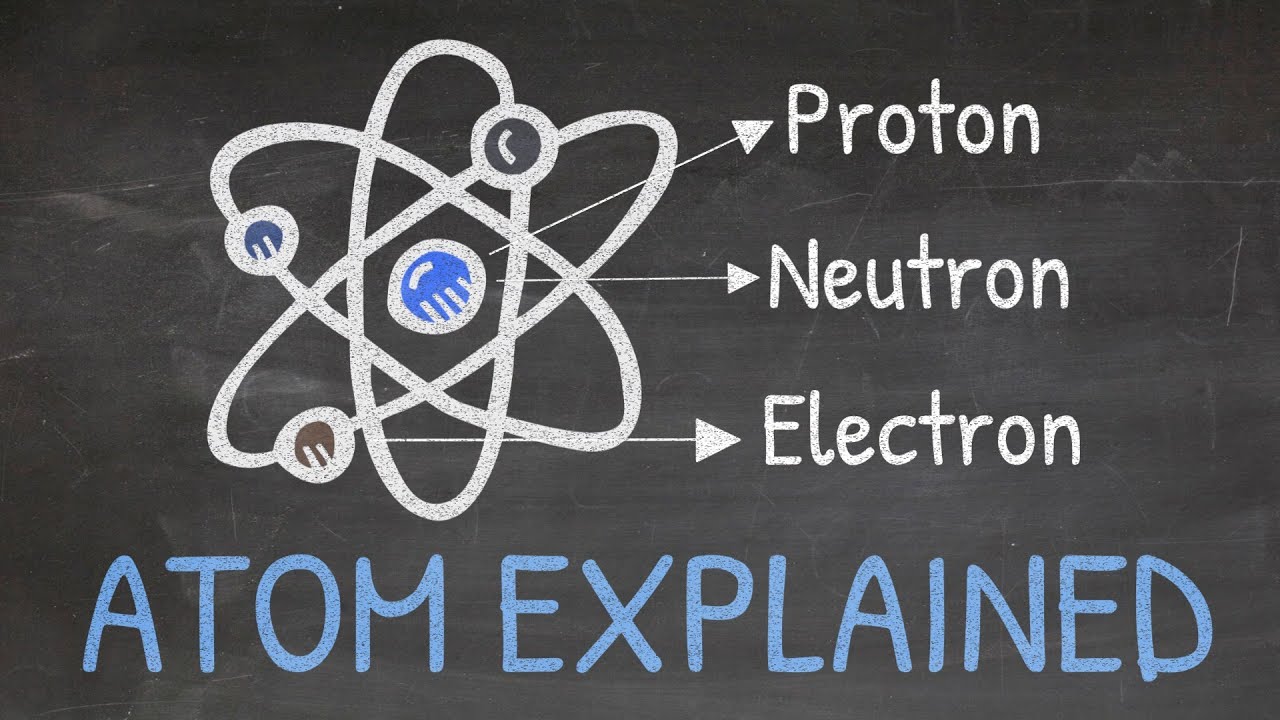
- 🌟 The modern atomic structure model includes protons, neutrons, and electrons, with the nucleus containing the protons and neutrons and electrons orbiting around it.
- 🔍 Electrons are not small balls but are better described by their wave-like nature, following quantum mechanics principles.
- 📊 The boundary of an atom is approximately 1 to 5 angstroms, with the nucleus being much smaller, around 10^-4 angstroms.
- ⚽ Using the football field analogy, if an atom were the size of a football field, the nucleus would be the size of a marble or thumb in the center.
- 🔋 Electrons are considered indivisible in current understanding, while protons and neutrons are composed of quarks.
- 💫 The charge of electrons and protons are equal in magnitude but opposite in sign (-1 for electrons and +1 for protons), while neutrons have no charge.
- 📈 The atomic mass unit (amu) is used to express the masses of subatomic particles, with 1 amu equal to 1.66054 × 10^-24 grams.
- 🎯 Atomic number represents the number of protons in the nucleus, which determines the element, while isotopes vary in the number of neutrons.
- 🔌 Atoms are electrically neutral with equal numbers of protons and electrons, but ions have a net charge due to the loss or gain of electrons.
- 🚀 The strong nuclear force holds the nucleus together, overcoming the electrostatic repulsion between protons, but very heavy nuclei can be unstable and radioactive.
Q & A
What is the modern view of atomic structure?
-The modern view of atomic structure includes understanding that atoms consist of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, while electrons orbit around the nucleus. Electrons are not considered as small balls but rather exhibit wave-like behavior, as described by quantum mechanics.
How do electrons behave in relation to the atom's nucleus?
-Electrons do not behave as small balls orbiting the nucleus like planets. Instead, they exhibit wave-like properties and can be found at various probabilities around the nucleus, with the highest probability at a certain distance known as the orbital radius.
What is the role of quarks in the structure of protons and neutrons?
-Protons and neutrons are composed of smaller particles called quarks. Each proton contains three quarks arranged in a specific way, while a neutron also consists of three quarks but arranged differently. The type and arrangement of quarks determine the identity and charge of the particle.
How is the atomic number related to the number of protons in an atom?
-The atomic number is directly equivalent to the number of protons in an atom. It determines the identity of the element, as each element has a unique number of protons.
What is an isotope and how does it relate to the number of neutrons in an atom?
-Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. This results in atoms with the same chemical properties but varying masses due to the difference in neutron count.
Why can't you create an infinitely heavy version of an element?
-You cannot create an infinitely heavy version of an element because as you add more neutrons to the nucleus, the strong nuclear force, which holds the nucleus together, becomes less effective due to the increasing size of the nucleus. Eventually, the nucleus becomes unstable and radioactive, undergoing decay.
What is the significance of the mass number in relation to the atom's mass?
-The mass number is the sum of the protons and neutrons in an atom's nucleus. It is a measure of the atom's mass since the mass of electrons is negligible compared to that of protons and neutrons. The mass number thus gives an indication of how heavy the atom is.
How does the strong nuclear force affect the stability of an atom's nucleus?
-The strong nuclear force is responsible for holding the nucleus together despite the electrostatic repulsion between protons. However, as more neutrons are added, the nucleus becomes larger and the strong force has a harder time maintaining its grip, leading to instability and potential radioactive decay.
What is the role of the electron's wave-like nature in chemical reactions?
-The wave-like nature of electrons, as described by quantum mechanics, provides the probability of where an electron might be found around the nucleus. This understanding is crucial for predicting how atoms will interact and react chemically, as it involves the movement and sharing of electrons between atoms.
How does the atomic mass unit (amu) simplify the representation of atomic mass?
-The atomic mass unit (amu) is a unit of mass that simplifies the representation of atomic mass by providing a scale where the mass of a proton or neutron is approximately 1 amu. This allows chemists to ignore the very small and complex values of their masses in grams or kilograms and focus on the relative differences in mass between different atoms and isotopes.
Outlines
🔬 Modern Atomic Structure: An Overview
This video script introduces the concept of modern atomic structure, moving beyond the historical discovery of atoms to present a contemporary understanding. It emphasizes the importance of protons, neutrons, and electrons in forming the atom's structure and discusses the electron behavior, challenging the simplified model of electrons as mere particles. The lesson aims to correct misconceptions about atomic structure by providing a more accurate picture of how electrons truly behave, utilizing a blend of summary and spoiler alerts to prepare viewers for a journey into atomic and quantum mechanics.
🌌 Exploring the Atom: Components and Characteristics
The script delves into the detailed composition of atoms, focusing on the helium atom as an example. It describes the atom's nucleus, composed of protons and neutrons, and the surrounding electrons, emphasizing the atom's mostly empty space. The discussion extends to the wave-like nature of electrons, contrasting this with the traditional particle view. By exploring the quantum mechanics perspective, the narrative prepares the viewer for a deeper understanding of atomic behavior, underscoring the vast emptiness within atoms and the misleading nature of simplified atomic models.
🔍 The Quantum World: Beyond Simple Models
This section expands on the complex nature of electrons, challenging the simplified view of them as particles orbiting the nucleus. It introduces quantum mechanics as a more accurate framework for understanding electron behavior, presenting electrons as waves rather than discrete particles. The discussion covers the dual wave-particle nature of both light and matter, illustrating the limitations of classical physics in explaining atomic and subatomic phenomena. By bridging the gap between early atomic models and contemporary quantum theory, the script invites viewers to rethink their understanding of atomic structure.
🌐 Atoms and Isotopes: Defining Characteristics
Focusing on the practical aspects of atomic theory, this part of the script explains the concept of atomic number, mass number, and isotopes, using the helium atom as an example. It clarifies how atoms are defined by their number of protons and how variations in the number of neutrons lead to different isotopes of the same element. This section serves as a bridge between theoretical quantum mechanics and its application in chemistry, emphasizing the importance of these concepts in understanding chemical behavior and the structure of the periodic table.
🧪 Chemical Identity and Atomic Interaction
The script transitions to a discussion on how atomic structure influences chemical reactions, highlighting the role of electrons in forming chemical bonds and the indirect influence of protons. By explaining ions, electronegativity, and how atoms achieve neutrality, this segment offers a comprehensive look at the chemical behavior of atoms based on their electronic structure. It underscores the balance between protons and electrons in determining the properties of atoms and molecules, setting the stage for more detailed explorations of chemical reactions.
⚛️ Understanding Atomic Mass and Charge
This section provides a detailed breakdown of the charges and masses of protons, neutrons, and electrons, introducing the concept of atomic mass units (AMUs) for measuring atomic and subatomic particles. It explores the similarities and differences in mass between protons and neutrons and discusses the negligible mass of electrons in comparison. This foundational knowledge is critical for understanding atomic weight and the role of these particles in the atom's structure and stability, offering a quantitative perspective on atomic composition.
📊 Atomic Numbers, Mass Numbers, and Isotopes
Delving deeper into atomic structure, this part of the script examines the significance of atomic numbers and mass numbers in identifying elements and their isotopes. It explains how the atomic number determines an element's identity, while variations in the mass number due to differences in neutron count result in isotopes. Through examples, it illustrates how isotopes of the same element have identical chemical properties but differ in mass, introducing the concept of isotopes in a way that connects atomic theory with practical observations in chemistry.
🔄 Isotopes and Their Role in Chemistry
This segment focuses on illustrating the concept of isotopes through carbon's various forms. It explains how isotopes, while chemically identical, differ in mass due to variations in their neutron numbers. By discussing carbon-12, carbon-13, and carbon-14, the script highlights the natural abundance of isotopes and their significance in fields such as radiometric dating. This detailed examination of isotopes emphasizes their importance in understanding atomic mass, nuclear stability, and the practical implications for science and technology.
🌟 Radioactivity and the Stability of Isotopes
Concluding the exploration of atomic structure, this final section addresses the stability of isotopes and the phenomenon of radioactivity, using carbon-14 as a case study. It discusses how the balance of forces within the nucleus affects an isotope's stability and explains the process of radioactive decay in terms of nuclear reactions. This part bridges the gap between atomic theory and nuclear chemistry, providing insights into the practical applications and implications of isotopic variation, including radiometric dating and the study of ancient artifacts.
Mindmap
Keywords
💡atomic structure
💡protons
💡neutrons
💡electrons
💡atomic number
💡mass number
💡isotopes
💡quantum mechanics
💡nuclear force
💡radioactivity
💡atomic mass unit (amu)
Highlights
The lesson focuses on the modern atomic structure, moving from historical discoveries to the current understanding of atoms.
Protons, neutrons, and electrons are the fundamental components of an atom, with the nucleus containing protons and neutrons and electrons orbiting around it.
Electrons are not small balls but are better understood as wave-like entities, following quantum mechanics principles.
The atomic structure model we commonly use is a simplification; electrons do not orbit in fixed paths but exist in probability clouds around the nucleus.
The size of an atom is incredibly small, measured in angstroms, with the nucleus being thousands of times smaller than the overall atom.
The number of protons in an atom determines its element, while the number of neutrons can vary, leading to different isotopes of the same element.
Isotopes have the same chemical properties due to the same number of protons but can differ in mass because of a different number of neutrons.
The atomic number represents the number of protons in an atom, which is equal to the number of electrons in a neutral atom.
The mass number is the sum of protons and neutrons in an atom's nucleus, with isotopes having different mass numbers due to varying neutron counts.
The strong nuclear force holds the nucleus together, overcoming the electrostatic repulsion between protons.
Nature doesn't allow for infinitely heavy atoms as the strong force has a maximum strength at a certain size, beyond which the nucleus becomes unstable and radioactive.
Carbon-14 is an isotope that is radioactive and is used in radiometric dating to determine the age of organisms.
The lesson emphasizes the importance of understanding the atomic structure and the concept of isotopes for further studies in chemistry.
The electron's charge is -1, the proton's charge is +1, and the neutron has no charge; these charges are crucial for atoms to be electrically neutral.
The mass of a proton or neutron is approximately 1 atomic mass unit (amu), while the electron's mass is significantly smaller, around 0.0005 amu.
The lesson provides a detailed explanation of how the number of protons, neutrons, and electrons contribute to the atom's identity and stability.
The strong force's ability to hold the nucleus together diminishes as the nucleus grows larger, leading to instability and radioactivity in heavy isotopes.
Transcripts
Browse More Related Video

Atomic Structure full topic
Protons, Neutrons and Electrons Explained - what's the difference?

Introduction to the atom | Chemistry of life | Biology | Khan Academy

Basic Atomic Structure: A Look Inside the Atom

Isotopes and Isobars | Atoms and Molecules | Don't Memorise

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
5.0 / 5 (0 votes)
Thanks for rating: