Acid/Base Dissociation Constant
TLDRThis video script delves into the concepts of acid and base dissociation constants, distinguishing between strong and weak acids and bases. It explains the process of dissociation in water, where strong acids and bases dissociate completely, while weak ones do not. The script then introduces the method of writing dissociation constant expressions and demonstrates how to calculate these constants through a sample problem involving acetic acid, revealing its weak acidic nature with a calculated dissociation constant.
Takeaways
- 📚 The difference between strong and weak acids and bases lies in their degree of dissociation in water; strong acids and bases dissociate completely, while weak ones only partially dissociate.
- 💧 Strong acids and bases dissociate 100%, resulting in a higher concentration of ions in solution, whereas weak acids and bases only partially dissociate, leading to fewer ions.
- 🔍 The strength of an acid or base can be determined by calculating its dissociation constant, with a smaller constant indicating a weaker acid or base.
- 📊 The dissociation constant for acids is represented as Ka, and for bases, it is represented as Kb.
- 🌟 The dissociation expression is written by comparing the concentration of the products (ions) to the concentration of the reactants (undissociated molecules).
- 🍲 Hydrochloric acid (HCl) is an example of a strong acid, dissociating completely into hydrogen ions (H+) and chloride ions (Cl-).
- 🍶 Acetic acid (CH3COOH) is an example of a weak acid, partially dissociating into hydrogen ions (H+) and acetate ions (CH3COO-).
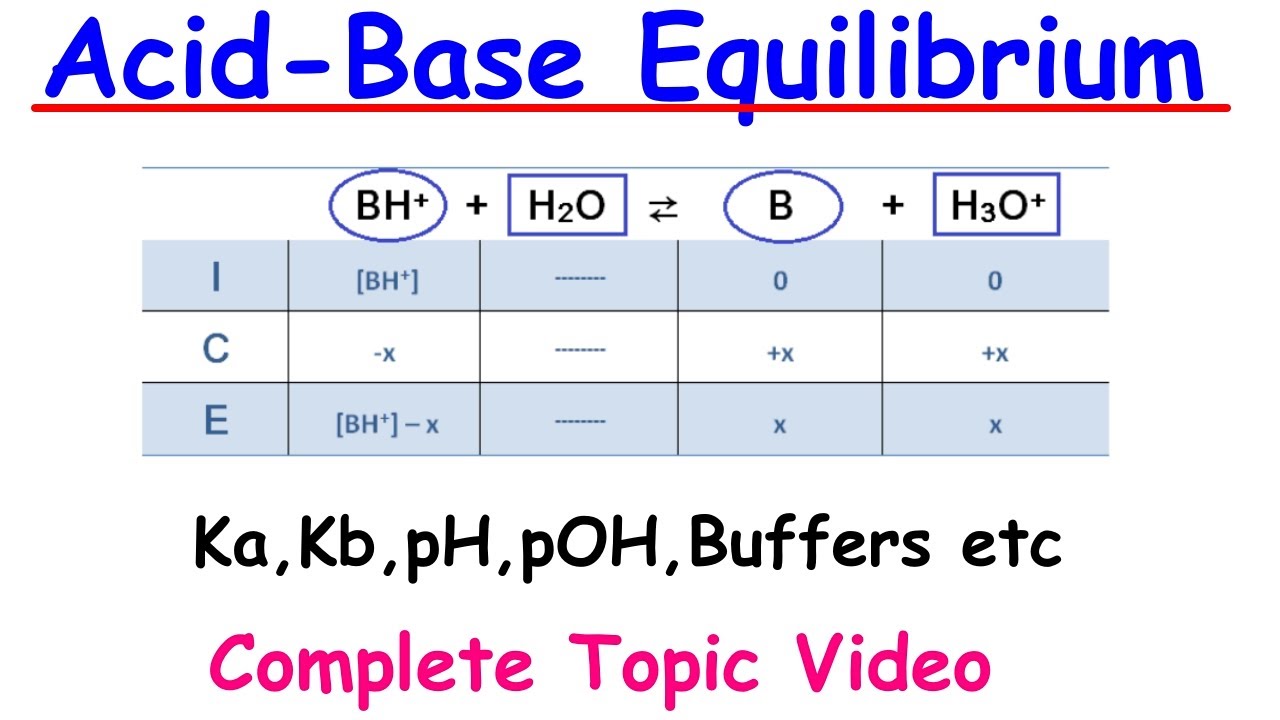
- 🔄 The process of calculating the dissociation constant involves setting up an ICE (Initial, Change, Equilibrium) chart to track the concentrations of reactants and products at different stages of dissociation.
- 📈 The equilibrium expression is used to find the Ka or Kb value by considering the concentrations at equilibrium, which is the final state after dissociation has occurred.
- 🧪 A.1 molar solution of acetic acid with a hydrogen ion concentration of 1.32 * 10^-3 M was used as an example to demonstrate the calculation of the acid dissociation constant.
- 📝 The calculated Ka value for acetic acid in the example was 1.77 * 10^-5, indicating that it is a weak acid due to the small dissociation constant value.
Q & A
What is the primary difference between a strong acid and a weak acid?
-A strong acid dissociates 100% in water, splitting into hydrogen ions and an anion, whereas a weak acid only partially dissociates, resulting in fewer hydrogen ions in solution.
How can we determine the strength of an acid or base?
-We can determine the strength of an acid or base by calculating its dissociation constant (Ka for acids and Kb for bases). The smaller the dissociation constant, the weaker the acid or base.
What happens when a base dissociates in water?
-When a base dissociates in water, it splits into a metal ion (usually not affecting acidity or basicity) and a hydroxide ion. The more hydroxide ions present, the stronger the base.
What is the general formula for the dissociation constant of an acid?
-The general formula for the dissociation constant of an acid is Ka = [H+][A-] / [HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the anion, and [HA] is the concentration of the undissociated acid.
What does the dissociation constant expression for a base look like?
-The dissociation constant expression for a base is Kb = [M+][OH-] / [MOH], where [M+] is the concentration of the metal ion, [OH-] is the concentration of hydroxide ions, and [MOH] is the concentration of the undissociated base.
How does the value of the dissociation constant (Ka or Kb) relate to the strength of an acid or base?
-A larger dissociation constant value indicates a stronger acid or base. A smaller value suggests a weaker acid or base that does not dissociate as completely in solution.
What is the chemical formula for acetic acid?
-The chemical formula for acetic acid is CH3COOH.
In the given problem, what is the concentration of hydrogen ions in a 0.1 molar solution of acetic acid?
-In the given problem, the concentration of hydrogen ions in a 0.1 molar solution of acetic acid is 1.32 * 10^-3 molar.
How does the ICE chart (Initial, Change, Equilibrium) help in solving dissociation constant problems?
-The ICE chart helps in visualizing the initial concentrations, the changes that occur during dissociation, and the equilibrium concentrations of all species involved in the reaction. It aids in calculating the dissociation constant by comparing the concentrations at equilibrium.
What is the calculated Ka value for acetic acid in the provided example?
-The calculated Ka value for acetic acid in the provided example is 1.77 * 10^-5.
Why is a small Ka value indicative of a weak acid like acetic acid?
-A small Ka value indicates that the acid does not dissociate completely in solution, which is characteristic of a weak acid. Acetic acid, with a Ka of 1.77 * 10^-5, is a weak acid because it does not ionize well in water.
How can we use the dissociation constant to predict the behavior of an acid or base in solution?
-By knowing the dissociation constant, we can predict how much an acid or base will ionize in solution. A larger constant indicates a stronger acid or base that will dissociate more completely, while a smaller constant indicates a weaker acid or base that will remain largely undissociated.
Outlines
📚 Introduction to Acid and Base Dissociation Constants
This paragraph introduces the concept of acid and base dissociation constants, differentiating between strong and weak acids and bases. It explains that a strong acid or base dissociates completely in water, resulting in a 100% ionization, while a weak acid or base only partially dissociates. The paragraph also outlines the process of writing dissociation constant expressions and mentions that the strength of an acid or base can be determined by calculating these constants. The example of hydrochloric acid as a strong acid and its dissociation process is provided, along with the general formula for the dissociation constant of an acid (Ka).
🧪 Calculation of Acid Dissociation Constant: Acetic Acid Example
This paragraph delves into the calculation of the acid dissociation constant (Ka) using acetic acid as an example. It begins by establishing the initial concentration of acetic acid and the changes that occur during dissociation, highlighting the 1:1 stoichiometric ratio. The paragraph introduces the concept of an ICE (Initial, Change, Equilibrium) chart to track these changes and demonstrates how to use the equilibrium expression to solve for Ka. The calculated Ka value for acetic acid is given as 1.77 * 10^-5, indicating that it is a weak acid with limited dissociation.
Mindmap
Keywords
💡Acid and Base
💡Dissociation
💡Dissociation Constant
💡Strong Acid
💡Weak Acid
💡Hydrogen Ion Concentration
💡Anion
💡Sodium Ion
💡Hydroxide Ion
💡Equilibrium
💡ICE Chart
Highlights
The video discusses acid and base dissociation constants, providing foundational knowledge on the topic.
The difference between strong and weak acids and bases is explained, focusing on their dissociation behavior in water.
A strong acid is defined as one that dissociates 100% in solution, resulting in a high concentration of H+ ions.
Weak acids only partially dissociate, leading to a lower concentration of H+ ions compared to strong acids.
Bases are discussed in terms of their dissociation into metal ions and hydroxide ions, with the latter being key to their basic nature.
The strength of a base is determined by the concentration of hydroxide ions in solution.
Dissociation constants, represented by K_a for acids and K_b for bases,量化 the strength of an acid or base.
The smaller the dissociation constant, the weaker the acid or base.
Hydrochloric acid is given as an example of a strong acid with a detailed explanation of its dissociation process.
Acetic acid is used as an example of a weak acid, with a step-by-step explanation of its partial dissociation.
The method for writing dissociation constant expressions for both acids and bases is outlined.
A practical problem is solved to demonstrate the calculation of the acid dissociation constant, using acetic acid as the example.
The ICE (Initial, Change, Equilibrium) chart is introduced as a tool for visualizing and calculating changes in concentration during dissociation.
The concept of stoichiometry is applied to understand the 1:1 ratio in the dissociation of acetic acid into H+ and its anion.
The calculation results in an acid dissociation constant (K_a) for acetic acid, which is used to determine its strength as an acid.
A small K_a value for acetic acid indicates that it is a weak acid with limited dissociation.
The video provides a comprehensive understanding of how to work with acid-base dissociation constants, including theoretical concepts and practical applications.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: