AP Chemistry Unit 5 Part 1 Review: Reaction Kinetics
TLDRThe video script offers a concise crash course on chemical reaction kinetics, emphasizing the concept of motion in chemical reactions. It explains that kinetics measures the speed of reactions, not the current state, and introduces the rate law, which is dependent on the rate constant, concentration, and order of the reactants. The script also addresses common challenges in determining the orders of reactants from data tables and provides mnemonic devices for remembering the relationships between orders and their graphical representations. Additionally, it highlights the unique characteristic of first-order reactions, where the half-life remains constant over time, offering helpful memory aids for this concept.
Takeaways
- 🌟 Chemical kinetics is the study of the rates at which chemical reactions occur, focusing on the speed of reactions rather than the reactions' current state.
- 📈 The rate of a chemical reaction can be expressed as the change in moles per second, similar to how velocity is measured in meters per second.
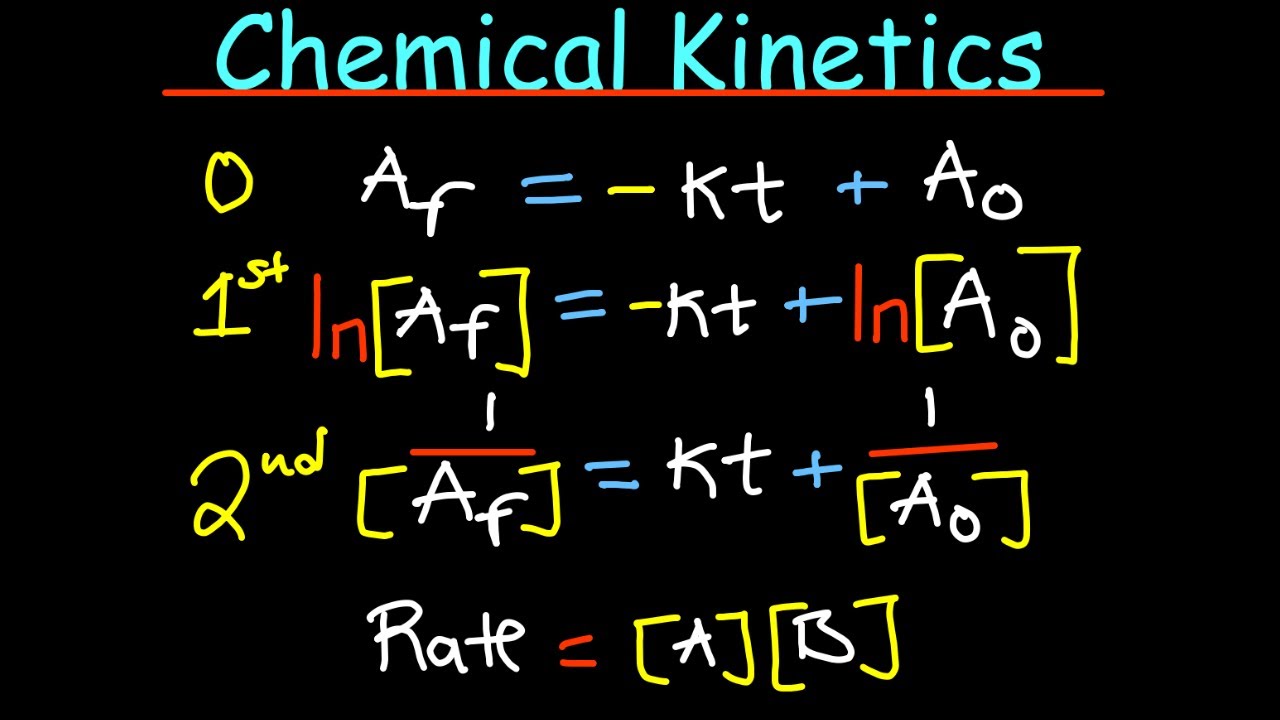
- 📚 The rate law is given by the formula rate = k[A]^m[B]^n, where k is the rate constant, [A] and [B] are the concentrations of reactants, and m and n are the orders with respect to each reactant.
- 🔥 The rate constant (k) is dependent only on temperature and is termed 'constant' because it does not change with concentration or the progress of the reaction.
- 🔄 The overall order of a reaction is the sum of the orders of each reactant (m + n) and does not directly correlate with the stoichiometric coefficients.
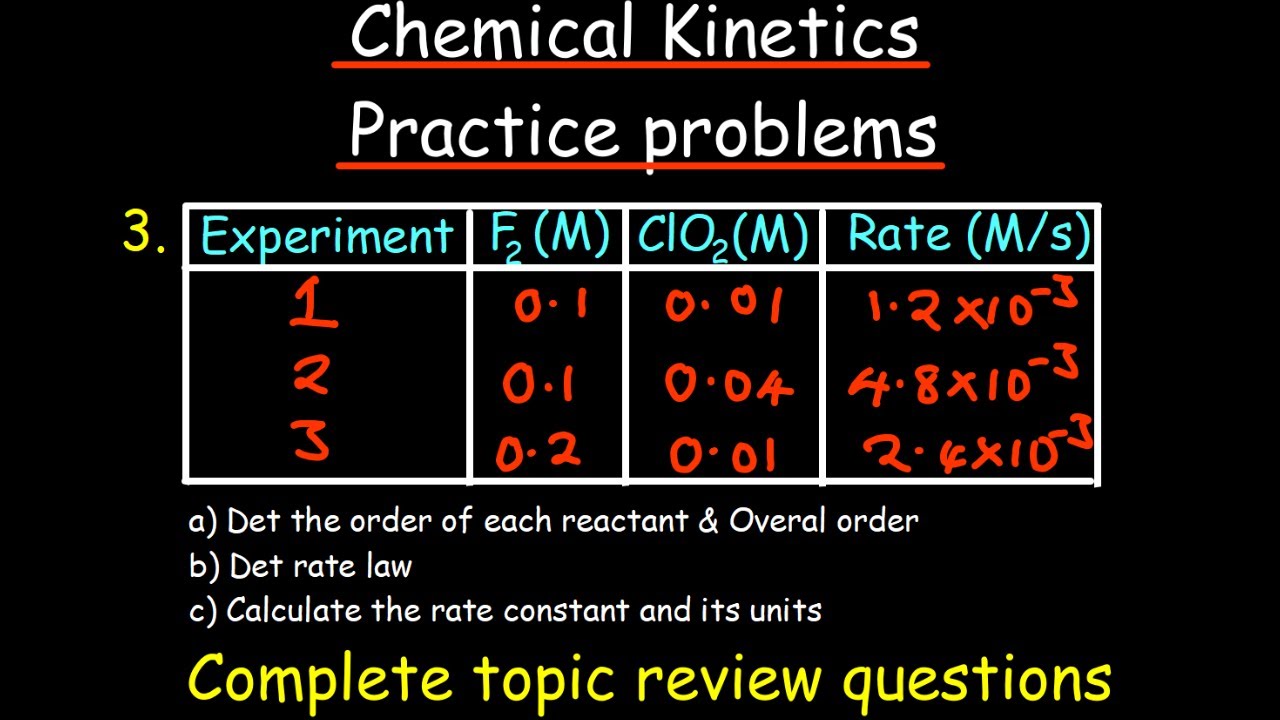
- 🧐 Determining the orders of reaction (m and n) involves analyzing data and comparing reaction rates under different initial concentrations to find the relationship between concentration and rate.
- 📊 When dealing with reaction rate data, comparing the ratios of rates can help determine the orders of the reactants without having to solve complex equations.
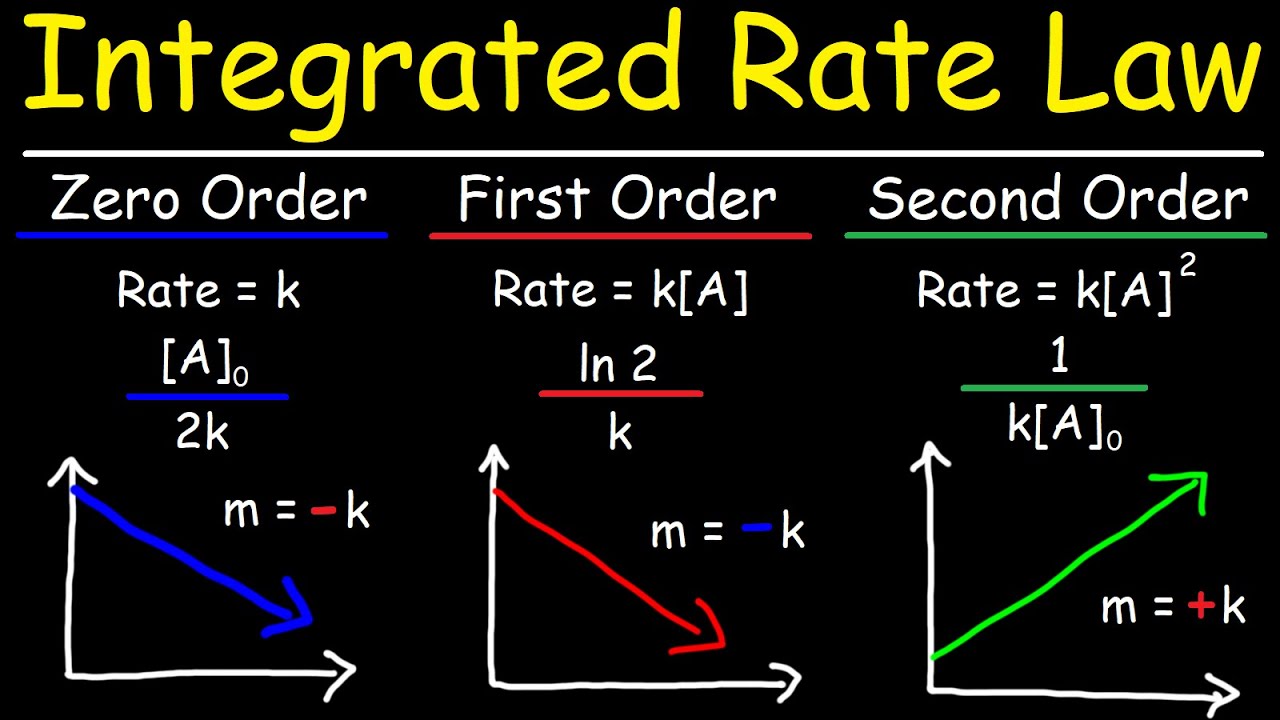
- 📈 Understanding the order of reactions is crucial as it affects how reaction rates are represented graphically, with different orders leading to different graphical behaviors (e.g., linear for zeroth order, logarithmic for first order).
- 🎓 A helpful mnemonic for remembering the graphical representation of orders is to associate the order with the function applied to the concentration: no function for zeroth order, natural logarithm (ln) for first order, and square for second order.
- 🕰️ First-order reactions have a constant half-life, which does not change over time, unlike other orders where the half-life can vary with concentration.
- 🚀 To memorize concepts in chemical kinetics, creating personal associations and mnemonics can be highly effective, even if they seem unconventional or 'stupid' to others.
Q & A
What is the primary focus of chemical kinetics?
-The primary focus of chemical kinetics is to determine the speed at which a reaction occurs, not the current state of the reactants or products.
How is the rate of a chemical reaction expressed?
-The rate of a chemical reaction is expressed as the change in moles per second, which is analogous to the rate of position change in meters per second.
What does the rate law equation represent in the context of chemical kinetics?
-The rate law equation represents the relationship between the rate of a reaction and the concentrations of the reactants, as well as the rate constant and the orders of the reactants.
What is the rate constant (k) in the rate law equation, and how does it relate to temperature?
-The rate constant (k) is a value that is only dependent on temperature. It remains constant if the temperature does not change.
What is the significance of the orders (m and n) in the rate law equation?
-The orders (m and n) indicate how the rate of the reaction is affected by the concentration of each reactant. They do not depend directly on the stoichiometric coefficients in the balanced chemical equation unless it's an elementary reaction.
How can one determine the orders of the reactants in a chemical reaction?
-To determine the orders of the reactants, one can compare different rates of reaction and analyze how changes in concentration affect these rates, using the method of initial rates or other techniques.
What is the method for determining the order of a reactant from a graph?
-The order of a reactant can be determined from a graph by looking at the relationship between the reaction progress and the time. For example, a straight line on a graph indicates a zeroth-order reaction, while a graph of 1/[A] vs. time indicates a first-order reaction.
What is a mnemonic to remember the relationship between the order of a reaction and its graph?
-A mnemonic to remember the relationship is to associate the order with a logarithmic function (Ln). For example, a first-order reaction is associated with the natural logarithm of the concentration (Ln[A]), and a second-order reaction is associated with 1/[A]^2.
Why is the first-order reaction special in chemical kinetics?
-The first-order reaction is special because its half-life does not change over time, unlike other orders of reactions.
How can one remember the relationship between the half-life and the first-order reaction?
-One can remember this by associating the natural logarithm (Ln) with the exponential function, which is related to decay processes like radioactive decay. The half-life (t1/2) can be calculated as ln(2) divided by the rate constant (k).
What is the advice given in the script for memorizing chemical kinetics concepts?
-The advice given is to create random associations that are unique or memorable, even if they seem silly, as these can help in better retaining the information.
Outlines
📚 Introduction to Chemical Reaction Kinetics
This paragraph introduces the concept of chemical reaction kinetics, emphasizing that kinetics is about the rate of reactions, not the current state. It explains that kinetics is akin to kinetic energy, focusing on the speed of reactions. The speaker clarifies that when dealing with kinetics, the goal is to determine an expression for the rate of reaction, which is the speed at which moles change, analogous to the rate of position change in meters per second. The paragraph also introduces the rate equation, stating that the rate of reaction can be expressed as K (the rate constant) times the concentration of reactants raised to the power of their respective orders (m and n). It highlights that m and n do not directly depend on the equation unless it's an elementary reaction. The speaker then discusses the challenges of determining the orders of reactants from given data and provides a method for solving for the orders, emphasizing the importance of comparing rates and looking for patterns.
🧠 Memorization Techniques for Reaction Orders
The second paragraph delves into the challenges of memorizing reaction orders and provides creative mnemonic devices to aid recall. It starts by discussing the difficulty of remembering the order of reactions from graphs and introduces the concept of using 'karate chops' as a memory aid. The speaker then offers a more effective method for remembering by associating the order with the natural logarithm (Ln) and the number 8, suggesting that the order is somehow related to the Ln of the concentration. The paragraph also touches on the special case of first-order reactions, highlighting that their half-life remains constant over time. The speaker shares personal mnemonic devices for remembering the half-life formula, Ln 2 over K, and encourages viewers to create their own associations to better memorize the concepts.
Mindmap
Keywords
💡Chemical Reaction Kinetics
💡Rate Constant (k)
💡Concentration
💡Reaction Orders (m, n)
💡Rate Law
💡Half-Life
💡Elementary Reaction
💡Activation Energy
💡Mnemonics
💡Exponential Functions
💡Temperature
Highlights
Kinetics is the study of the speed of chemical reactions, not the current state of the reaction.
The rate of a chemical reaction can be determined by the change in moles over time, similar to how speed is measured in meters per second.
The rate of reaction is expressed as K times the concentration of reactants raised to the power of their respective orders.
The order of a reaction with respect to a substance does not depend directly on the coefficients in the equation unless it's an elementary reaction.
To find the orders of the reactants, one can compare different rates of reaction and use algebraic methods to solve for the orders.
The rate constant is only dependent on temperature and remains constant if the temperature is unchanged.
The overall reaction order is the sum of the orders of each reactant.
A method to remember the orders of reactions is to associate them with the functions applied to the concentration, such as Ln for first order.
First-order reactions have a half-life that does not change over time, which is a unique characteristic.
The half-life of a first-order reaction can be calculated using the formula Ln 2 divided by the rate constant.
The concept of half-life is related to exponential functions and decay processes, which is why first-order reactions have a constant half-life.
Memorization techniques can involve creating associations, even nonsensical ones, to help remember complex concepts.
The video provides practical tips for understanding and memorizing chemical kinetics concepts, which can be beneficial for students studying AP Chemistry.
The讲师 uses a straightforward approach to explain complex concepts, making it easier for students to grasp the fundamentals of chemical reaction kinetics.
The讲师introduces a method to determine reaction orders from graphs, which is crucial for analyzing experimental data in kinetics.
The讲师emphasizes the importance of understanding the relationship between the rate constant, reaction orders, and the rate law in chemical kinetics.
The讲师provides a clear explanation of how to calculate the rate of a chemical reaction, which is a fundamental skill in studying kinetics.
The讲师explains how to use algebraic techniques to solve for reaction orders, which is an essential skill for more advanced studies in chemical kinetics.
The讲师highlights the unique properties of first-order reactions, such as the constant half-life, which is a key concept in understanding reaction dynamics.
The讲师shares mnemonic devices to help remember the order of reactions, which can be particularly useful for students struggling with the material.
The讲师's teaching style is engaging and aims to simplify complex topics, making the content more accessible to a wider audience.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: