GCSE Physics - Alpha, Beta and Gamma Radiation #33
TLDRThis educational video delves into the world of nuclear radiation, explaining the four primary types: alpha particles, beta particles, gamma rays, and neutron emission. It highlights the unique characteristics of each, such as their composition, charge, ionizing capability, and penetrating power. Alpha particles, with a helium nucleus composition, are highly ionizing but can be stopped by paper. Beta particles, essentially electrons, are moderately ionizing and can penetrate materials like aluminum. Gamma rays, electromagnetic waves, are weakly ionizing and require dense materials like lead to stop them. Lastly, neutron emission is a simple process of neutron release for stability, adding to the understanding of radioactive decay and its implications.
Takeaways
- 📊 Isotopes are different forms of an element with the same number of protons but varying numbers of neutrons.
- 🌟 Only one or two isotopes of an element are stable; the rest are unstable and undergo radioactive decay.
- 💫 Radioactive decay involves the emission of particles to increase stability in unstable isotopes.
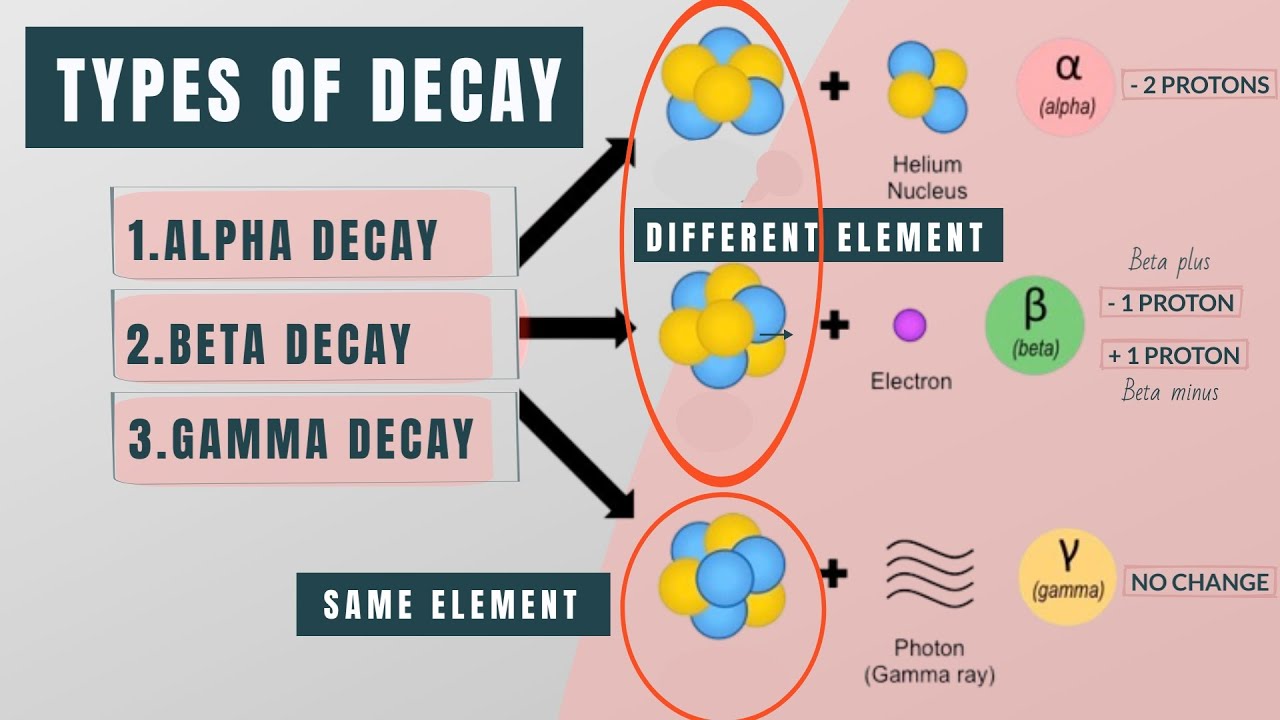
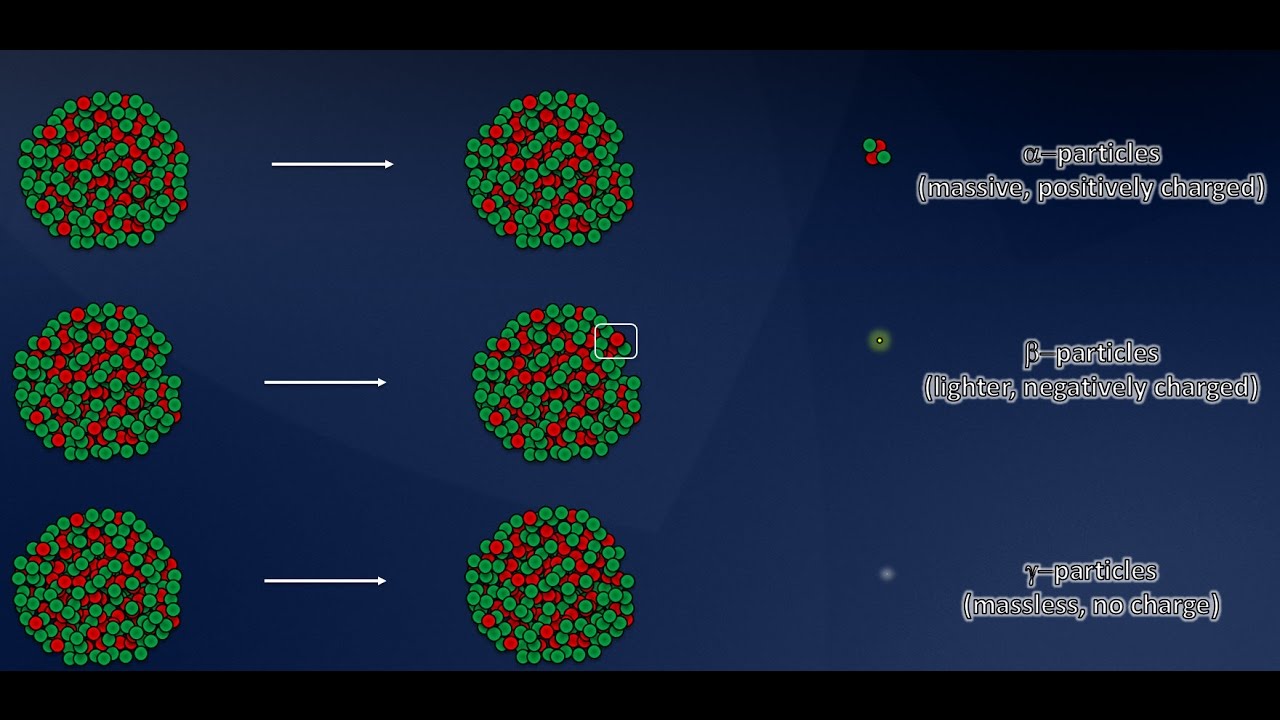
- 🚀 Alpha particles consist of two protons and two neutrons, similar to a helium nucleus, and carry a +2 charge.
- 🥁 Alpha particles are large, highly ionizing, but cannot penetrate deeply; they are stopped by a sheet of paper.
- 🤖 Beta particles are electrons with a -1 charge, resulting from the decay of a neutron into a proton and an electron.
- 🌀 Beta particles are small, moderately ionizing, and can penetrate several meters of air or a few millimeters of aluminum.
- 💥 Gamma rays are not particles but electromagnetic waves like light, often emitted after alpha or beta radiation.
- 🛡️ Gamma rays are weakly ionizing and can penetrate deeply, requiring thick lead or concrete to stop them.
- 🔄 Neutron emission occurs when a nucleus has too many neutrons, leading to increased stability by releasing a neutron.
- 📝 Recap: Alpha particles are stopped by paper, beta particles by aluminum, and gamma rays by lead or concrete.
Q & A
What are isotopes?
-Isotopes are different forms of an element that have the same number of protons but different numbers of neutrons.
Why do isotopes undergo radioactive decay?
-Unstable isotopes undergo radioactive decay to emit particles and become more stable.
What are the four types of nuclear radiation mentioned in the script?
-The four types of nuclear radiation are alpha particles, beta particles, gamma rays, and neutrons.
What is the composition of alpha particles?
-Alpha particles are composed of two protons and two neutrons, which is the same as the nucleus of a helium atom.
How do alpha particles interact with materials?
-Alpha particles are large and carry a double positive charge, making them strongly ionizing but unable to penetrate very far into materials, stopped by a single sheet of paper.
How are beta particles formed?
-Beta particles are formed when a neutron in an atom decays into a proton and an electron; the electron is then emitted at high speed.
What are the characteristics of beta particles?
-Beta particles are tiny, carry a single negative charge, are moderately ionizing, and can penetrate several meters of air or about five millimeters of aluminum.
What is the nature of gamma rays?
-Gamma rays are not particles but electromagnetic waves like light, often emitted after alpha or beta radiation to help the nucleus release extra energy.
How do gamma rays interact with materials?
-Gamma rays have no mass or charge, pass straight through materials with weak ionization, and require thick sheets of lead or multiple meters of concrete to be stopped.
What happens when a nucleus has too many neutrons?
-If a nucleus has too many neutrons, making it unstable, it can emit a neutron to increase stability.
How can the different types of nuclear radiation be distinguished based on their properties?
-They can be distinguished by their composition, charge, mass, ionizing capability, and penetrating power through materials.
Outlines
🔬 Understanding Radioactivity and Isotopes
This paragraph introduces the concept of isotopes, which are different forms of an element with the same number of protons but varying numbers of neutrons. It explains that only one or two isotopes of an element are stable, with the rest being unstable and prone to radioactive decay. The paragraph also sets the stage for a deeper exploration of four types of nuclear radiation in the video: alpha particles, beta particles, gamma rays, and neutrons, and how they ionize and penetrate materials.
🚀 Alpha Radiation: Charged Helium Nuclei
The paragraph delves into alpha radiation, describing it as composed of two protons and two neutrons, identical to a helium nucleus. Due to their large size and double positive charge, alpha particles are easily stopped by collisions with other molecules and cannot penetrate deeply into materials, being absorbed by a single sheet of paper. Despite their limited range, their strong ionizing ability allows them to dislodge electrons from atoms they collide with.
🥺 Beta Radiation: High-Speed Electrons
Beta radiation is discussed as consisting of high-speed electrons, which have a single negative charge and negligible mass. Unlike alpha particles, beta particles are not emitted from an atom's shells but are produced when a neutron decays into a proton and an electron, with the latter being emitted. Beta particles are moderately ionizing and can penetrate further into materials than alpha particles, requiring several meters of air or a few millimeters of aluminum to be stopped.
🌟 Gamma Rays: Electromagnetic Waves
Gamma rays are characterized as electromagnetic waves similar to light, often emitted alongside alpha or beta radiation as the nucleus releases excess energy. Unlike particles, gamma rays pass through materials with minimal interaction, making them weakly ionizing. They can travel long distances through air and require dense materials like thick sheets of lead or meters of concrete to be effectively stopped.
💫 Neutron Emission: Adjusting Nuclei Stability
The final type of radiation discussed is the emission of neutrons, which occurs when a nucleus has too many neutrons, leading to instability. To regain stability, the nucleus can expel a neutron. The paragraph serves as a brief recap, summarizing the key points about the different types of nuclear radiation covered in the video, including their composition, ionizing capabilities, and penetration depths.
Mindmap
Keywords
💡Isotopes
💡Radioactive Decay
💡Alpha Particles
💡Beta Particles
💡Gamma Rays
💡Ionizing Radiation
💡Penetrating Power
💡Nuclear Stability
💡Emission of Neutrons
💡Electromagnetic Waves
Highlights
Isotopes are different forms of an element with the same number of protons but varying numbers of neutrons.
Only one or two isotopes of an element are stable; the rest are unstable and can undergo radioactive decay.
Radioactive decay involves the emission of particles to stabilize an unstable isotope.
Alpha particles consist of two protons and two neutrons, similar to a helium nucleus.
Alpha particles have a +2 charge due to the presence of two positive protons and lack electrons.
Alpha particles are large and can't penetrate very far; they are stopped by a single sheet of paper.
Despite their size, alpha particles are highly ionizing due to their strong charge.
Beta particles are essentially electrons with a -1 charge and virtually no mass.
Beta particles are produced when a neutron decays into a proton and an electron within an atom.
Beta particles are moderately ionizing and can penetrate several meters in air or a few millimeters of aluminum.
Gamma rays are not particles but electromagnetic waves like light, often emitted alongside alpha or beta radiation.
Gamma rays are weakly ionizing and can penetrate long distances through materials.
To stop gamma rays, thick sheets of lead or multiple meters of concrete are required.
Neutron emission can occur when a nucleus has too many neutrons, leading to increased instability.
A neutron is simply thrown out by an unstable nucleus to enhance stability.
The video provides a comprehensive overview of the four types of nuclear radiation and their properties.
Understanding the characteristics of alpha, beta, gamma, and neutron radiation is crucial for nuclear science and applications.
Transcripts
Browse More Related Video

Alpha decay | High school chemistry | Khan Academy
Different types of decay | Alpha vs. Beta vs. Gamma decay | Visual Explanation

What is radiation?
A Brief Introduction to Alpha, Beta and Gamma Radiation

Types of Nuclear Radiation

Alpha Particles, Beta Particles, Gamma Rays, Positrons, Electrons, Protons, and Neutrons
5.0 / 5 (0 votes)
Thanks for rating: