What is radiation?
TLDRThe video script, presented by Julie from the Canadian Nuclear Safety Commission, offers an insightful overview of radiation, a phenomenon that is both ubiquitous and essential in various aspects of modern life. It explains that radiation is the emission of energy in the form of waves or particles, distinguishing between non-ionizing radiation, such as microwaves and cell phones, and ionizing radiation, like X-rays and cosmic rays. The script delves into the atomic structure, highlighting the nucleus as the source of nuclear energy and the cause of radioactivity when an atom becomes unstable due to an imbalance of neutrons. This instability leads to radioactive decay, which can manifest in three forms: alpha, beta, and gamma, each with varying levels of penetration and distance traveled. The concept of half-life is introduced to illustrate how radioactive substances diminish over time, a principle applied in nuclear medicine, exemplified by the use of Technetium-99m in diagnostic imaging. The video concludes with the Canadian Nuclear Safety Commission's role in regulating nuclear substances for peaceful purposes and safeguarding public health and the environment, encouraging viewers to seek further information from their trusted source.
Takeaways
- 🧬 Radiation is the release of energy in the form of waves or particles, which can be low-level (non-ionizing) or high-level (ionizing).
- 🔬 Atoms are the building blocks of all matter, consisting of a nucleus with protons and neutrons, and electrons orbiting the nucleus.
- ⚛️ The nucleus contains energy, and when an atom has an unstable number of neutrons, it becomes radioactive, forming a radioisotope.
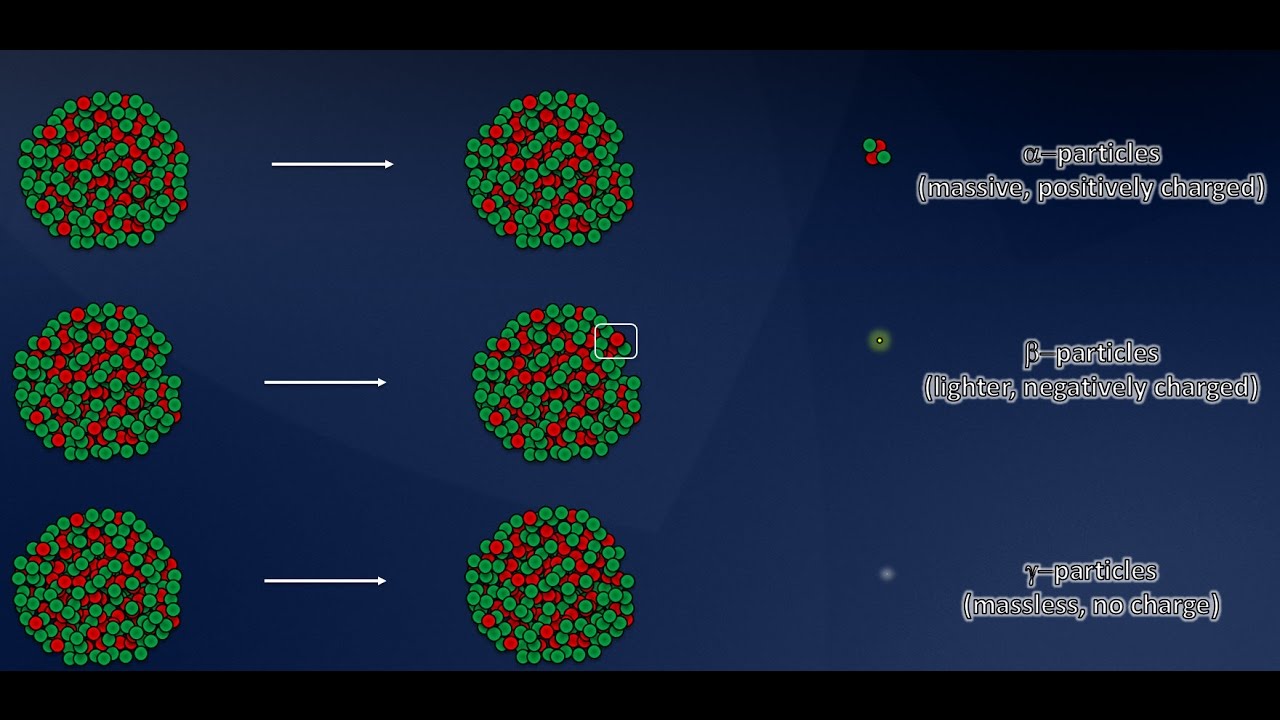
- ➗ Radioactive decay is the process by which unstable atoms release energy to become stable again, and it occurs in three main forms: alpha, beta, and gamma.
- 🚀 Alpha particles are heavy and have a short range, beta particles are lighter and travel further, and gamma radiation is a wave that travels the farthest.
- ⏳ The half-life of a radioisotope is the time it takes for half of the radioactive atoms to decay, and it can range from seconds to billions of years.
- 🏥 In nuclear medicine, radioisotopes like Technetium-99m, which has a short half-life, are used for diagnostic imaging with gamma cameras.
- 🍬 The example of jellybeans illustrates how the number of radioactive atoms decreases with each half-life, until nearly all radioactivity is gone after 24 hours.
- 🛡️ The Canadian Nuclear Safety Commission regulates the use of nuclear substances and materials in Canada, ensuring they are used peacefully and do not harm health or the environment.
- 🌐 The Canadian Nuclear Safety Commission is a trusted source for information on nuclear safety and can be found online at nuclearsafety.gc.ca, on YouTube, and Facebook.
- 🎓 Understanding radiation and its applications, such as in medicine, is crucial for appreciating the role it plays in various aspects of modern life.
Q & A
What is radiation?
-Radiation is the release of energy in the form of moving waves or streams of particles, which can be low-level like microwaves and cell phones, or high-level like X-rays or cosmic rays.
What are the two main types of radiation?
-The two main types of radiation are non-ionizing radiation, which includes microwaves and cell phones, and ionizing radiation, which includes X-rays and cosmic rays.
What are the components of an atom's nucleus?
-The nucleus of an atom is made up of protons, which carry a positive charge, and neutrons, which have no charge.
What is a radioisotope?
-A radioisotope is an unstable atom that has too many or too few neutrons, which causes it to be radioactive.
What is radioactive decay?
-Radioactive decay is the process by which an unstable atom releases energy to become stable again.
Name the three main types of radioactive decay.
-The three main types of radioactive decay are alpha, beta, and gamma. Alpha particles are heavy and travel short distances, beta particles are lighter and travel further, and gamma radiation is a wave that travels the farthest.
What is the half-life of a radioisotope?
-The half-life of a radioisotope is the time it takes for half of the radioactive atoms to decay.
How is Technetium-99m used in nuclear medicine?
-Technetium-99m is injected into patients and emits gamma radiation, which allows a gamma camera to take pictures of the patient's insides for diagnostic purposes.
Why is the short half-life of Technetium-99m ideal for medical tests?
-The short half-life of Technetium-99m, which is 6 hours, makes it ideal for medical tests because it reduces the patient's exposure to radiation and allows for quick diagnostic results.
Who regulates the nuclear sector in Canada?
-The Canadian Nuclear Safety Commission regulates the use of nuclear substances and materials in Canada, ensuring they are used for peaceful purposes and protecting health, safety, and the environment.
How can one find more information about the Canadian Nuclear Safety Commission?
-More information about the Canadian Nuclear Safety Commission can be found on their website at nuclearsafety.gc.ca, or by following them on YouTube or Facebook.
What is the role of the Canadian Nuclear Safety Commission in protecting the public and the environment?
-The Canadian Nuclear Safety Commission is responsible for regulating the use of nuclear materials to ensure they are used for peaceful purposes, and for protecting public health and safety as well as the environment.
Outlines
📡 Introduction to Radiation
The video introduces the concept of radiation, explaining that it involves the release of energy in the form of waves or particles, which can be either non-ionizing (like microwaves and cell phones) or ionizing (like X-rays or cosmic rays). It delves into the atomic structure, highlighting the nucleus composed of protons and neutrons, and electrons orbiting the nucleus. The video discusses how an imbalance in the number of neutrons can make an atom unstable or 'radioactive,' leading to the formation of radioisotopes. It also explains the process of radioactive decay, which helps unstable atoms achieve stability, and outlines the three main types of decay: alpha, beta, and gamma. The half-life of a radioisotope is introduced as the time it takes for half of the radioactive atoms to decay, with examples given from seconds to billions of years. The video concludes with an example of how half-lives are used in nuclear medicine, specifically with Technetium-99m, which has a short half-life of 6 hours and is used for diagnostic imaging.
Mindmap
Keywords
💡Radiation
💡Atom
💡Nucleus
💡Radioisotope
💡Radioactive Decay
💡Half-Life
💡Ionizing Radiation
💡Non-Ionizing Radiation
💡Canadian Nuclear Safety Commission
💡Technetium-99m
💡Gamma Radiation
💡Nuclear Medicine
Highlights
Radiation is the release of energy in the form of moving waves or streams of particles.
There are two types of radiation: non-ionizing (like microwaves and cell phones) and ionizing (like X-rays or cosmic rays).
Atoms are the microscopic building blocks of all matter in the universe.
The nucleus of an atom contains protons and neutrons, and is the source of tremendous energy.
An unstable atom, or radioisotope, can become radioactive if it has too many or too few neutrons.
Radioactive atoms undergo radioactive decay to become stable again, releasing energy in the process.
There are three main types of radioactive decay: alpha, beta, and gamma.
Alpha particles are heavy and travel short distances, beta particles are lighter and travel further, and gamma radiation travels the farthest.
The half-life of a radioisotope is the time it takes for half of the radioactive atoms to decay.
Half-lives can range from a fraction of a second to billions of years.
In nuclear medicine, doctors use radioisotopes like Technetium-99m, which has a short half-life, for diagnostic tests.
Technetium-99m emits gamma radiation, allowing gamma cameras to take images of a patient's insides.
After 24 hours, almost all radioactivity from Technetium-99m is gone due to decay and the body's natural processing.
The Canadian Nuclear Safety Commission regulates the use of nuclear substances and materials for peaceful purposes.
The Commission ensures the protection of health, safety, and the environment in relation to nuclear materials.
The Canadian Nuclear Safety Commission is a trusted source for information on nuclear safety.
The Commission can be found online at nuclearsafety.gc.ca, on YouTube, and on Facebook for further information.
Transcripts
Browse More Related Video

GCSE Physics - Alpha, Beta and Gamma Radiation #33

Types of Nuclear Radiation

Nuclear Chemistry: Crash Course Chemistry #38
A Brief Introduction to Alpha, Beta and Gamma Radiation

Nuclear Reactions, Radioactivity, Fission and Fusion
Radioactivity, Half-Life & Inverse Square Law - GCSE & A-level Physics (full version)
5.0 / 5 (0 votes)
Thanks for rating: