Thermodynamics: Crash Course Physics #23
TLDRThis video explores the science behind the Drinking Bird toy, using it as an intriguing example to explain the first and second laws of thermodynamics. It demystifies how the toy mimics perpetual motion through a cycle of dipping and bobbing, powered by heat transfer and changes in internal energy, rather than defying physics. The video further delves into thermodynamic processes—isovolumetric, isobaric, isothermal, and adiabatic—and their roles in energy transfer and work within systems. It also introduces the concept of entropy, illustrating how the universe's inherent disorder increases over time, showcasing the principles of thermodynamics in everyday phenomena.
Takeaways
- 😀 The first law of thermodynamics describes energy transfers via heat and work, equaling change in internal energy
- 😮💨 Heat can be converted to work and vice versa; this connects to conservation of energy
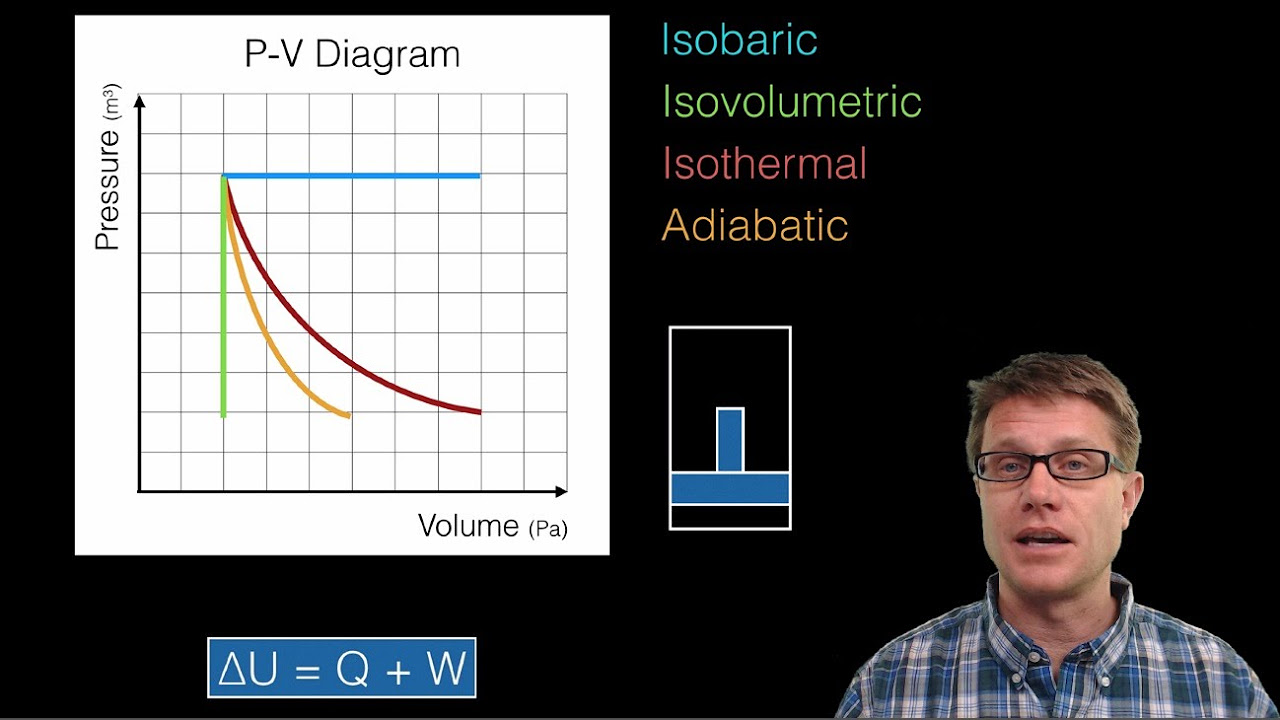
- 😎 Four thermodynamic processes: isovolumetric, isobaric, isothermal, adiabatic; properties like temperature, volume change
- 🤓 Isovolumetric: volume constant, adding/removing heat changes pressure and temperature
- 🧐 Isobaric: pressure constant, adding heat increases volume, temperature; can do work
- 🌡️ Isothermal: temperature constant; heat added expands volume, does work; calculus needed
- ❄️ Adiabatic: no heat transfer; work done equals negative change in internal energy
- 😈 Second law: heat flows spontaneously from hotter to colder; related to increasing entropy
- 🏋️ Entropy: disorder of a system; tends to increase for whole universe
- 💧 Drinking bird toy shows first and second laws: heat flows out, gas condenses, entropy increases
Q & A
What is the Drinking Bird toy, and how does it work?
-The Drinking Bird is a toy that appears to drink water from a cup autonomously. It functions based on a cycle of head dipping, cooling by evaporation, condensation creating a partial vacuum, and the resulting motion that keeps it bobbing. This cycle relies on the fluid inside the bird that has a low boiling point, allowing for easy phase changes with slight temperature variations.
Why is the Drinking Bird not considered a perpetual motion machine?
-The Drinking Bird is not a perpetual motion machine because it requires external energy to continue its motion, specifically the water for the evaporation and cooling process. Perpetual motion machines are impossible according to the laws of physics, as they would violate the first law of thermodynamics which states that energy cannot be created or destroyed.
What does the first law of thermodynamics state?
-The first law of thermodynamics, also known as the law of energy conservation, states that the change in internal energy of a closed system is equal to the heat transferred to the system minus the work done by the system. It emphasizes that energy can neither be created nor destroyed, only transformed or transferred.
How is work related to heat in thermodynamic processes?
-In thermodynamics, work and heat are interconnected forms of energy transfer. Work done by a system results in a loss of heat, and work done on a system results in a gain of heat. These interactions lead to changes in the system's internal energy.
What are the four basic types of thermodynamic processes?
-The four basic types of thermodynamic processes are isovolumetric (constant volume), isobaric (constant pressure), isothermal (constant temperature), and adiabatic (no heat exchange). Each type involves holding one property constant while allowing others to change, affecting the system's thermodynamic properties.
How does an isobaric process work, and what makes it interesting?
-In an isobaric process, pressure is held constant while heat is added or removed, allowing the volume of the gas to change. This process is interesting because it can do work, as the change in volume causes the gas to move a piston, translating thermal energy into mechanical work.
What distinguishes isothermal processes from other thermodynamic processes?
-Isothermal processes maintain a constant temperature by slowly exchanging heat or volume, allowing other properties to adjust. They require calculating work by taking the integral of pressure with respect to volume, considering the changing pressure, and result in no change in internal energy for an ideal gas.
What is entropy, and how does it relate to the second law of thermodynamics?
-Entropy represents the disorder within a system, and the second law of thermodynamics states that in any process, the total entropy of the universe will increase. This law explains why heat flows from hotter to colder bodies and underpins the irreversible nature of real-life processes.
Why can't entropy decrease in a closed system without affecting its surroundings?
-If entropy decreases in a system, the second law of thermodynamics requires that the entropy of the surroundings increase by a greater amount, ensuring the total entropy of the universe still increases. This principle explains phenomena like water freezing in a freezer while the surrounding kitchen warms up.
How do adiabatic processes differ from other thermodynamic processes?
-Adiabatic processes allow no heat flow into or out of the system, but still enable changes in the system's internal energy and volume. The work done in these processes directly impacts the internal energy, illustrating the first law of thermodynamics without heat exchange.
Outlines
🔬 Understanding the Drinking Bird and the First Law of Thermodynamics
This paragraph introduces the concept of the Drinking Bird toy as a seemingly perpetual motion machine, which leads into an exploration of the first law of thermodynamics. It explains how thermodynamics aims to describe energy transfer, specifically through work and heat, and how these processes affect a system's internal energy. The law is formulated as the change in internal energy being equal to the heat added to the system minus the work done by the system. The paragraph clarifies the significance of positive and negative values in this context and emphasizes that in a closed system, changes in internal energy can only result from work and heat. This principle underscores the conservation of energy and explains why the Drinking Bird, which operates through a cycle of evaporation and condensation driven by temperature changes, is not a perpetual motion machine but rather a clever demonstration of thermodynamic principles.
🌀 Delving into Thermodynamic Processes and the Second Law
This paragraph expands on the first law of thermodynamics by detailing the types of thermodynamic processes (isovolumetric, isobaric, isothermal, and adiabatic) and how they relate to changes in a system's properties while emphasizing the second law of thermodynamics and the concept of entropy. It explains how isothermal and adiabatic processes operate under constant temperature and no heat exchange conditions, respectively, and their implications for work and internal energy. The paragraph also introduces entropy as a measure of disorder, illustrating its role in determining the direction of heat flow and the overall increase in the universe's entropy over time. It concludes by connecting these concepts back to the Drinking Bird toy, showcasing it as an example of these laws in action and highlighting the broader implications for understanding heat, work, and entropy in the physical world.
Mindmap
Keywords
💡thermodynamics
💡heat
💡work
💡first law of thermodynamics
💡second law of thermodynamics
💡entropy
💡isovolumetric process
💡isobaric process
💡isothermal process
💡adiabatic process
Highlights
Introduction of the Drinking Bird toy as a seemingly perpetual motion machine.
Explanation of why the Drinking Bird is not a perpetual motion machine, grounded in the laws of physics.
Introduction to the first law of thermodynamics and its application in describing energy transfer.
Description of work and heat as processes of energy transfer.
Explanation of the internal energy change in a system, highlighting the connection between work, heat, and total energy.
The fundamental equation of the first law of thermodynamics representing the relationship between internal energy, heat, and work.
Clarification on the signs of Q and W in the context of energy transfer.
Emphasis on the first law of thermodynamics as a statement of energy conservation.
Mechanism of the Drinking Bird's motion explained through thermodynamic principles.
Introduction to four basic thermodynamic processes: isovolumetric, isobaric, isothermal, and adiabatic.
Explanation of isovolumetric processes and their characteristics.
Description of isobaric processes and how they allow for work through expansion or compression.
Understanding isothermal processes and the unique consideration of temperature and heat reservoirs.
Insights into adiabatic processes, where no heat is transferred, yet work and internal energy changes occur.
Introduction to the second law of thermodynamics and the concept of entropy, emphasizing spontaneous heat flow and disorder.
Practical implications of thermodynamic laws demonstrated through everyday phenomena, such as making ice and the behavior of gases.
Final reflection on the perpetual motion of the Drinking Bird as an educational tool for understanding thermodynamic laws.
Transcripts
Browse More Related Video
Thermodynamics and P-V Diagrams

The First Law of Thermodynamics: Internal Energy, Heat, and Work

23. The Second Law of Thermodynamics and Carnot's Engine

First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry

Entropy intuition | Thermodynamics | Physics | Khan Academy

Wayne Myrvold: Thermodynamics: The Basics
5.0 / 5 (0 votes)
Thanks for rating: