Different types of decay | Alpha vs. Beta vs. Gamma decay | Visual Explanation
TLDRThis video script, presented by a Belgian nuclear medicine resident, explores the three primary types of radioactive decay: alpha, beta, and gamma. Alpha decay involves the emission of helium nuclei, which are significant but have low penetration power, posing a health risk primarily if inhaled or ingested. Beta decay, on the other hand, emits high-energy electrons and can be stopped by simple barriers like clothing or aluminum, posing lesser risks. Lastly, gamma decay releases high-energy photons that penetrate deeply, impacting treatment methods in oncology and sterilization processes. Each type alters the atomic structure, transforming elements and impacting their surroundings.
Takeaways
- 🚀 **Alpha Decay**: A nucleus emits an alpha particle, which is a helium nucleus with 2 protons and 2 neutrons, resulting in the original atom becoming a different element.
- 🔬 **Alpha Particle Characteristics**: Alpha particles are energetic but have low penetration power and can be stopped by a sheet of paper or human skin, posing a health risk if ingested or inhaled.
- ☢️ **Health Effects of Alpha Decay**: Alpha radiation damages DNA, creates reactive free radicals, reduces white blood cell count, and targets organs like liver, kidneys, and bone marrow, causing rapid damage.
- 🌟 **Beta Decay Overview**: Beta decay involves the emission of beta particles, which are high-energy electrons, and can travel further than alpha particles but are less ionizing.
- ➕➖ **Types of Beta Decay**: Beta-plus decay involves a proton turning into a neutron, while beta-minus decay involves a neutron turning into a proton, with the release of beta particles and neutrinos.
- 🏥 **Medical Applications of Beta Decay**: Beta radioisotopes are used in brachytherapy to treat cancer and in PET scans for medical imaging.
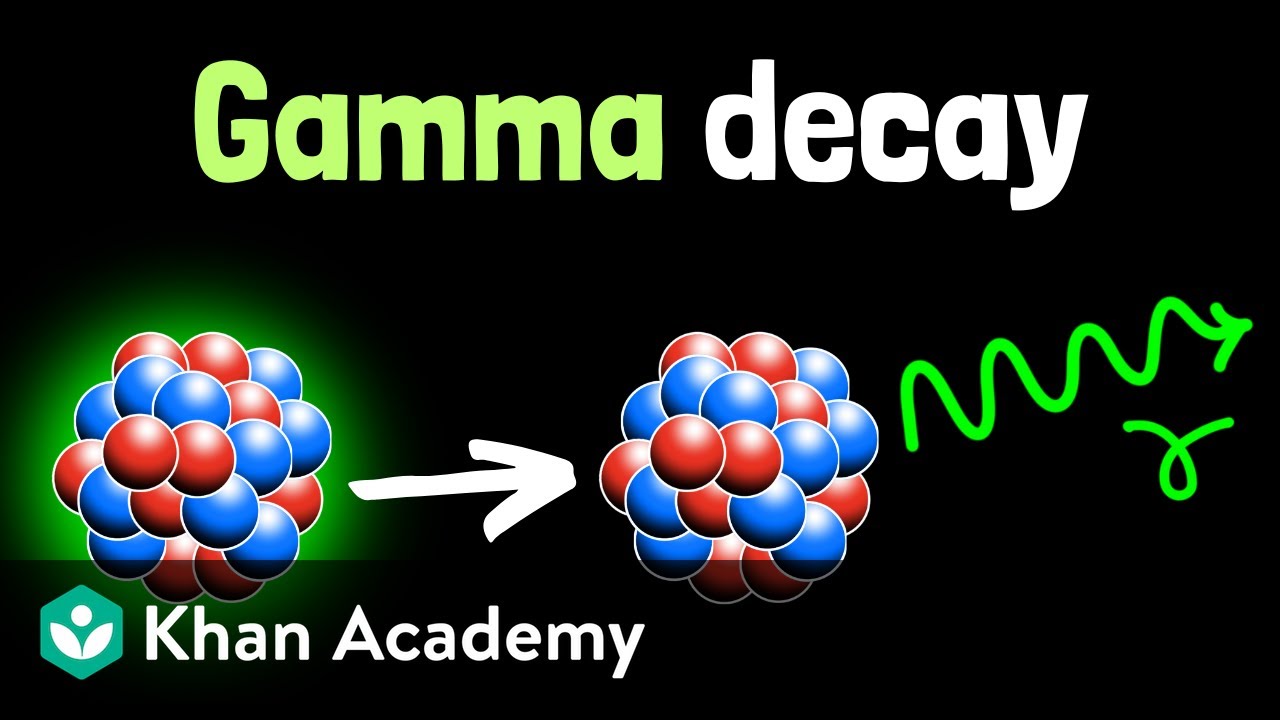
- 🧬 **Gamma Decay Distinction**: Unlike alpha or beta decay, gamma decay involves the release of high-energy electromagnetic radiation (gamma rays) without ejecting particles from the nucleus.
- 📡 **Gamma Radiation Properties**: Gamma rays have high frequency and energy, can penetrate most substances, and are absorbed by thick lead or concrete.
- ⚔️ **Gamma Rays in Medicine**: Used in oncology for treating tumors with gamma knives and in food preservation and medical equipment sterilization.
- ⚛️ **Cobalt 60 Application**: Produces low amounts of gamma radiation useful for killing bacteria, insects, and yeast in food without significant changes to its content.
- 🔢 **Decay Summary**: Alpha decay reduces the atomic number by 2 protons, beta decay involves a change in the number of protons (either loss or gain), and gamma decay does not change the atomic number or element.
- ☢️ **Radioactive Decay and Damage**: Alpha decay is less damaging, while gamma decay is the most damaging to living organisms.
Q & A
What are the three types of radioactive decay mentioned in the script?
-The three types of radioactive decay mentioned are alpha decay, beta decay, and gamma decay.
What is an alpha particle and how does it affect the atom that undergoes alpha decay?
-An alpha particle is a combination of two protons and two neutrons, equivalent to a helium nucleus. When an atom undergoes alpha decay, it loses four from its mass number (two from protons and two from neutrons) and two from its atomic number (from the two protons lost), resulting in the formation of a different element.
Why are alpha particles considered to have low penetration power?
-Alpha particles have low penetration power due to their massive size and the fact that they carry a double positive charge. They quickly lose energy and can be stopped by a sheet of paper or the outer layer of human skin.
What health risks are associated with alpha particles, and how was Alexander Litvinenko affected?
-Health risks associated with alpha particles include damage to DNA and the creation of reactive free radical ions, which can lead to further damage. If ingested or inhaled, they can cause a reduction in white blood cell count, increased susceptibility to infection, and damage to internal organs. Alexander Litvinenko was poisoned by polonium-210, an alpha emitter, which led to severe health issues and his eventual death.
How do beta particles differ from alpha particles in terms of size, stopping power, and ionizing power?
-Beta particles are electrons with high energy and are much smaller than alpha particles. They can travel further before being stopped, but they can be stopped by a layer of clothing or a sheet of aluminum foil. Their ionizing power is considerably smaller than that of alpha particles, by about 10 times.
What are the two main types of beta decay, and how do they differ in terms of the changes they cause in the atom?
-The two main types of beta decay are beta-plus decay and beta-minus decay. In beta-plus decay, a proton turns into a neutron, releasing a beta-plus particle and a neutrino, resulting in one less proton and one more neutron in the daughter atom. In beta-minus decay, a neutron turns into a proton, releasing a beta-minus particle and an electron antineutrino, resulting in one less neutron and one more proton in the daughter atom.
How is beta decay utilized in medical applications?
-Beta decay is utilized in medical applications such as brachytherapy, where beta radioisotopes are used to irradiate areas inside a patient to prevent the growth of certain cancers. Additionally, in positron emission tomography (PET), the emission of beta particles (positron emission) is used for medical scanning, with fluorine-18 being one of the isotopes routinely used in PET imaging.
What is unique about gamma decay compared to alpha and beta decay?
-Unlike alpha and beta decay, gamma decay does not involve the ejection of particles from the nucleus. Instead, a high-energy form of electromagnetic radiation, a gamma-ray photon, is released. Gamma decay does not change the structure or composition of the atom, meaning the atom does not become a different element.
How can gamma radiation be used in medicine, and what are its properties?
-Gamma radiation can penetrate most common substances, including metals, and is used in medicine to treat malignant tumors. In a controlled procedure known as a 'gamma knife,' multiple concentrated beams of gamma rays are focused directly onto a tumor to kill cancer cells. Gamma rays are also used to preserve food and sterilize medical equipment due to their ability to kill bacteria, insects, and yeast.
What are the differences in the damage caused by alpha, beta, and gamma decay to living organisms?
-Alpha decay, while ionizing, is the least damaging of the three types of decay because of its low penetration power. Beta decay can cause more damage due to its ability to travel further and its ionizing properties, but it is still less damaging than gamma decay. Gamma decay is the most damaging because it can penetrate deeply and deposit significant energy in tissues, causing severe biological harm.
How does the mass number and atomic number of an atom change during alpha and beta decay?
-During alpha decay, the mass number decreases by four and the atomic number by two, as the atom loses two protons and two neutrons. In beta decay, the mass number remains the same, but the atomic number changes: it decreases by one in beta-plus decay (proton to neutron conversion) and increases by one in beta-minus decay (neutron to proton conversion).
What is the role of neutrinos in beta decay, and why are they significant?
-Neutrinos are nearly massless, uncharged particles that are released alongside beta particles during beta decay. They play a role in conserving the overall energy and lepton number in the decay process. Neutrinos are significant because they are involved in weak nuclear interactions and have been instrumental in our understanding of the weak force and the Standard Model of particle physics.
Outlines
🔬 Understanding Radioactive Decay
This paragraph introduces the three primary types of radioactive decay: alpha, beta, and gamma decay. It emphasizes the importance of understanding these processes in nuclear physics. Alpha decay is described as the emission of an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. This decay reduces the mass number by four and the atomic number by two, resulting in a new element. Alpha particles are energetic but have low penetration power, making them a health risk only if ingested or inhaled. Beta decay involves the emission of beta particles, which are high-energy electrons. There are two types of beta decay: beta-plus, where a proton turns into a neutron, and beta-minus, where a neutron turns into a proton. Beta particles have higher penetration power than alpha particles but lower ionizing power. The paragraph also discusses the medical applications of beta decay in brachytherapy and PET scans.
📡 Gamma Decay and Its Applications
The second paragraph focuses on gamma decay, which is distinct from alpha and beta decay as it does not involve the ejection of particles from the nucleus. Instead, gamma decay releases a high-energy electromagnetic radiation known as a gamma-ray photon. Gamma rays are part of the electromagnetic spectrum but have higher frequency and energy than visible light, and they can penetrate most substances. The only materials capable of absorbing gamma radiation are thick lead and concrete. Gamma decay does not alter the atomic structure or composition, hence the atom remains the same element. Gamma rays are used in medicine, particularly in oncology, to treat tumors with a 'gamma knife' that focuses multiple beams onto the tumor to kill cancer cells. Additionally, gamma radiation is utilized for food preservation and sterilization of medical equipment, with Cobalt 60 being a source of low-level gamma radiation for these purposes. The paragraph concludes with a summary of the effects of radioactive decay on living organisms, noting that alpha decay is the least damaging and gamma decay the most.
Mindmap
Keywords
💡Alpha decay
💡Beta decay
💡Gamma decay
💡Radioactive decay
💡Half-life
💡Mass number
💡Atomic number
💡Ionizing radiation
💡Neutrino
💡Positron emission
💡Brachytherapy
Highlights
There are three types of radioactive decay: alpha decay, beta decay and gamma decay.
Alpha decay occurs when a nucleus emits an alpha particle, which is a helium nucleus.
The resulting atom loses four from its mass number and two from its atomic number, becoming a different element.
Alpha particles have low penetration power and can be stopped by a sheet of paper or human skin.
Ingested or inhaled alpha particles can damage DNA and cause health issues like bone marrow failure and hair loss.
Beta decay involves the emission of high-energy electrons known as beta particles.
Beta particles have higher penetration power than alpha particles but can be stopped by clothing or aluminum foil.
Beta-plus decay occurs when a proton turns into a neutron, while beta-minus decay is the reverse process.
Brachytherapy uses beta radioisotopes to irradiate and prevent the growth of cancerous cells.
Positron emission tomography (PET) uses the emission of beta particles for medical scanning.
Gamma decay involves the release of high-energy electromagnetic radiation in the form of gamma-ray photons.
Unlike alpha and beta decay, gamma decay does not change the structure or composition of the atom.
Gamma rays have high penetration power and can pass through most substances, requiring lead or concrete for absorption.
Gamma rays are used in medicine to treat malignant tumors with a 'gamma knife' procedure.
Cobalt 60 produces gamma radiation for preserving food and sterilizing medical equipment.
In alpha decay, the nucleus loses two protons. In beta decay, the nucleus loses a proton (beta plus) or gains a proton (beta minus).
Gamma decay does not change the number of protons, so the atom does not become a different element.
Radioactive decay can damage living things, with alpha decay being the least damaging and gamma decay the most damaging.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: