Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (68 of 92) What is the Wave Number k=?
TLDRThe video script delves into the concept of wave number (K), clarifying its definition as the number of radians per unit distance and its relation to wavelength. It explains how K is calculated by dividing 2π radians by the wavelength (λ), and further connects K to the momentum of a particle through Planck's constant (h-bar). The script illustrates that a lower wave number corresponds to a longer wavelength, using the example of particles approaching a step potential to demonstrate this relationship. Overall, the video aims to demystify wave number and its significance in wave physics.
Takeaways
- 🌊 Wave number (K) is defined as the number of radians per unit distance in a wave.
- 📏 Wavelength is equivalent to 360 degrees or 2π radians, which relates to the wave number.
- 🔢 K is calculated as 2π radians divided by the wavelength (λ) in meters.
- 📐 For a wave with a 4-meter wavelength, K equals π/2 radians per meter.
- 🌀 The wave number indicates the number of radians per meter, which corresponds to the wave's wavelength.
- 💫 K can also be seen as the momentum of a particle divided by h-bar (ħ), where h is Planck's constant.
- 🌱 As the wave number (K) decreases, the wavelength (λ) increases, and vice versa.
- 🎲 The wave number's change can be observed in quantum mechanics, such as in a particle approaching a step potential.
- 📈 A smaller wave number (K) results in a larger wavelength, which can affect particle transmission.
- 🔄 The relationship between wave number and wavelength is fundamental to understanding wave-particle duality.
- 📚 Understanding wave number is crucial for grasping various concepts in physics, including quantum mechanics.
Q & A
What is the definition of wave number (K)?
-The wave number (K) is defined as the number of radians per unit distance in a wave.
How is the wave number related to the wavelength?
-The wave number (K) is inversely proportional to the wavelength. It can be calculated as 2π radians divided by the wavelength (λ) in meters.
What does a wavelength of 360 degrees or 2π radians signify?
-A wavelength of 360 degrees or 2π radians signifies a full circle or a complete cycle of the wave.
How can one calculate the wave number for a wave with a wavelength of 4 meters?
-For a wave with a wavelength of 4 meters, the wave number (K) would be calculated as 2π divided by 4, which equals π/2 radians per meter.
What is the significance of the relationship between wave number and momentum in quantum mechanics?
-In quantum mechanics, the wave number (K) can be defined as the momentum (p) of a particle divided by h-bar (ħ), which is Planck's constant divided by 2π. This relationship connects the wave-like properties of particles with their momentum.
How does the wave number affect the wavelength of a particle?
-As the wave number decreases, the wavelength increases. This means that a smaller wave number corresponds to a larger wavelength for a particle.
What happens to the wave number when a particle transitions from one region to another with a different potential?
-When a particle transitions from one region to another, the wave number (K) on the right side will be smaller than on the left side if the particle is transmitted through a step potential.
What is the de Broglie wavelength?
-The de Broglie wavelength (λ) of a particle is given by the Planck's constant (h) divided by the product of 2π and the particle's momentum (p), represented as λ = h / (2πp). It describes the wavelength associated with a particle in quantum mechanics.
How can one determine the wavelength of a particle knowing its momentum?
-The wavelength of a particle can be determined by the formula λ = h / (MV), where h is Planck's constant, M is the mass of the particle, and V is its velocity.
What is the implication of a smaller wave number in the context of wave propagation?
-A smaller wave number implies a larger wavelength, which means that the wave covers more spatial extent per cycle as it propagates.
How does the concept of wave number help in understanding wave behavior?
-The concept of wave number helps in understanding the spatial frequency of waves, which is the number of cycles that occur over a certain distance. It provides insight into how waves with different wavelengths interact with their environment and other waves.
Outlines
🌊 Introduction to Wave Number
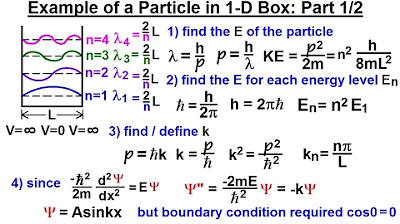
This paragraph introduces the concept of wave number (K), acknowledging its complexity and historical difficulty in understanding. It explains that wave number is defined as the number of radians per unit distance and relates this to the wavelength of a wave, which is equivalent to 360 degrees or 2π radians. The explanation continues with a practical example, showing how to calculate K for a wave with a wavelength of 4 meters, and further discusses the relationship between wave number and momentum of a particle, introducing the concept of de Broglie wavelength.
Mindmap
Keywords
💡Wave Number (K)
💡Wavelength
💡Radians
💡Momentum
💡h-bar (h/2 pi)
💡de Broglie Wavelength
💡Step Potential
💡Quantum Mechanics
💡Wave-Particle Duality
💡Planck's Constant (h)
💡Radiance
Highlights
The lecture focuses on explaining the concept of wave number, which can be confusing.
Wave number (K) is defined as the number of radians per unit distance.
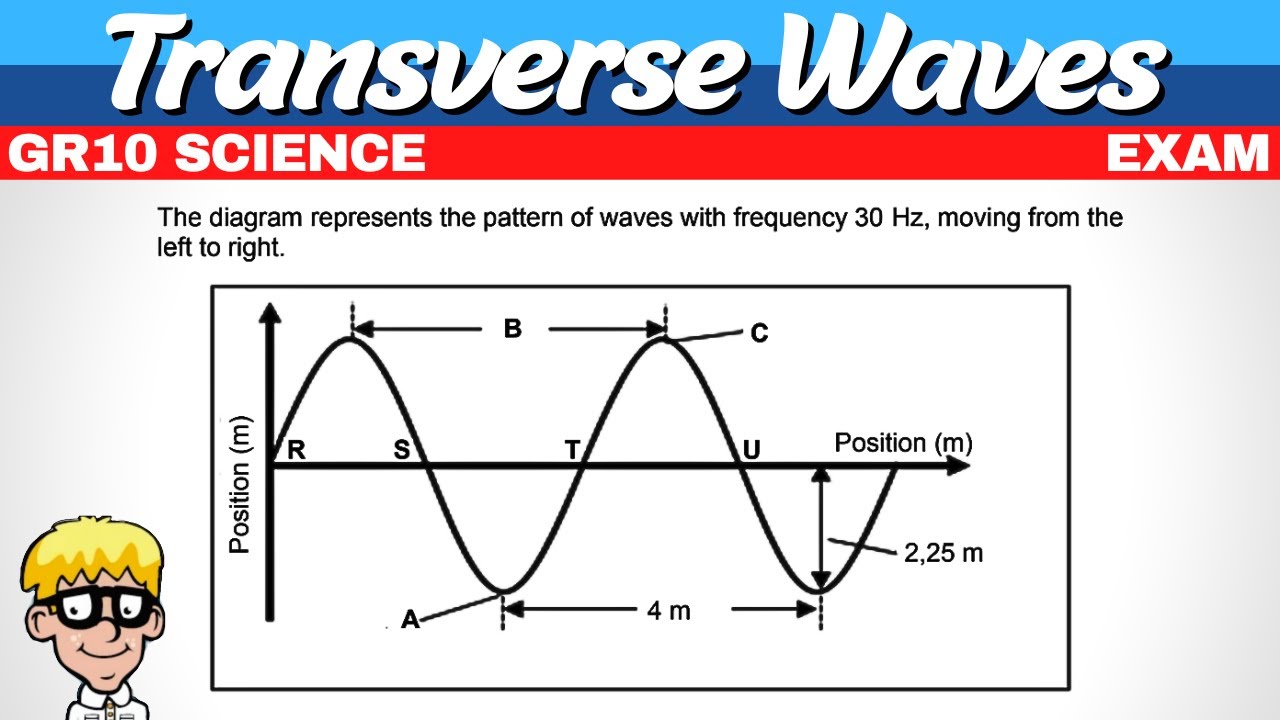
Wavelength is equivalent to 360 degrees or 2 pi radians.
The relationship between wavelength and wave number is derived by dividing the wavelength by 2 pi radians.
For a wave with a wavelength of 4 meters, the wave number K is calculated as pi over 2 radians per meter.
Wave number can also be understood as the momentum of a particle divided by h-bar.
The momentum is represented as M times V, where M is the mass and V is the velocity.
h-bar is defined as Planck's constant divided by 2 pi.
The wavelength of a particle can be defined as h-bar divided by the momentum (h/MV).
As the wave number decreases, the wavelength increases.
A smaller wave number corresponds to a larger wavelength.
In the context of a particle approaching a step potential, a decrease in wave number results in an increase in wavelength.
The wave number is fundamental in understanding wave behavior and particle transmission.
The concept of wave number is central to wave-particle duality and quantum mechanics.
The lecture provides a clear and detailed explanation of wave number, its calculation, and its significance.
The wave number is a key parameter in the study of wave phenomena and quantum mechanics.
Understanding wave number helps in the application of wave-particle duality principles in various scientific fields.
The lecture elucidates the connection between wave number, wavelength, and the behavior of particles in different potentials.
The wave number is a measure of how many radians are contained in a given distance, which is crucial for wave analysis.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: