2.4 Reference Table H (Vapor Pressure and Temperature)
TLDRThis video script offers a detailed explanation of Reference Table H, which illustrates the relationship between temperature and vapor pressure for four key liquids. It highlights how increasing temperature raises vapor pressure, allowing molecules to escape as gases. The script explains the significance of the 101.3 kPa line, which represents standard pressure and is used to determine normal boiling points. It also discusses how pressure changes affect boiling temperatures and the implications for intermolecular forces, providing insights into the relative strength of these forces by comparing vapor pressures at a fixed temperature.
Takeaways
- 📈 The script discusses reference table H, which contains a graph showing the relationship between temperature and vapor pressure for four different liquids.
- 🔍 As temperature increases, vapor pressure also increases, indicating that molecules with more kinetic energy are more likely to move and escape as gases.
- 🌡️ The graph includes a line at 101.3 kPa, which is the standard atmospheric pressure, and is used to determine the normal boiling points of the liquids.
- 💧 For water, the normal boiling point is 100 degrees Celsius at standard pressure, but this can change with varying atmospheric pressures.
- ⛰️ At lower atmospheric pressures, such as 70 kPa, water boils at a lower temperature, approximately 91 degrees Celsius.
- 🔬 The script emphasizes the importance of reading the graph accurately, especially the scales on the axes, to avoid misinterpretation of the data.
- 🔄 The table can be used to compare the vapor pressures of different liquids at the same temperature, indicating the relative strength of intermolecular forces.
- 🌡️ At 60 degrees Celsius, the order in which the liquids' vapor pressures are encountered on the graph reflects their relative ease of evaporation.
- 🧪 Ethanoic acid has a low vapor pressure at 60 degrees Celsius, indicating strong intermolecular forces, while propanone has a high vapor pressure, suggesting weaker forces.
- 🔑 The normal boiling points of different liquids at 101.3 kPa can be compared to understand the strength of their intermolecular forces, with propanone boiling at a lower temperature than water.
- 📚 The script concludes that table H is a valuable resource for understanding the relationship between temperature, vapor pressure, and intermolecular forces, and for making accurate readings and comparisons.
Q & A
What is the main purpose of the video script?
-The main purpose of the video script is to explain and discuss the reference table H, which illustrates the relationship between temperature and vapor pressure for different liquids.
What does the upward trend of the lines in table H indicate?
-The upward trend of the lines in table H indicates that as temperature increases, vapor pressure also increases, which is a basic principle that can be understood by a fourth grader.
How does the increase in temperature affect molecules in a liquid?
-As temperature increases, molecules gain more kinetic energy, which allows them to move faster and more of them can break free and exist as gases above their respective liquids.
What is the significance of the line drawn at 101.3 kPa in table H?
-The line at 101.3 kPa in table H is significant because it represents standard atmospheric pressure, and it is used to determine the normal boiling points of the liquids when the pressure is at this standard level.
What is the normal boiling point of water, and how does it relate to the standard pressure line in table H?
-The normal boiling point of water is 100 degrees Celsius, which is the temperature at which water boils when the pressure is at the standard pressure of 101.3 kPa.
How does a change in atmospheric pressure affect the boiling point of water?
-A decrease in atmospheric pressure results in a lower boiling point for water. For example, at 70 kPa, water boils at approximately 91 degrees Celsius instead of 100 degrees Celsius.
What can be inferred about the intermolecular forces of different liquids by comparing their vapor pressures at a specific temperature?
-By comparing vapor pressures at a specific temperature, one can infer the relative strength of intermolecular forces; a higher vapor pressure suggests weaker intermolecular forces, making it easier for the liquid to evaporate.
How can table H be used to compare the boiling points of different liquids at standard pressure?
-By looking at where each liquid's line intersects with the standard pressure line at 101.3 kPa, one can compare their boiling points and draw conclusions about the relative strength of their intermolecular forces.
What does the video script suggest about the relationship between vapor pressure and the ease of a liquid turning into a gas?
-The script suggests that a higher vapor pressure at a given temperature indicates that it is easier for the liquid to turn into a gas due to weaker intermolecular forces.
What precautions should be taken when using table H to ensure accurate readings?
-When using table H, one should be careful to match the scale on the graph with the values being read, ensuring that the increments on the x and y-axes are correctly interpreted to avoid misreading the data.
How can the information in table H be used to understand the effect of pressure on the boiling point of substances?
-Table H can be used to understand that by lowering the pressure, the temperature at which substances boil decreases, as demonstrated by the graph showing the relationship between pressure and boiling point for different liquids.
Outlines
📊 Understanding Reference Table H: Temperature and Vapor Pressure
This paragraph introduces Reference Table H, which graphically represents the relationship between temperature and vapor pressure for four different liquids. The script explains that as temperature increases, so does vapor pressure, indicating that molecules with more kinetic energy are more likely to escape as gases. The table is used to identify the normal boiling points of these liquids at standard atmospheric pressure (101.3 kPa). Additionally, the script discusses how changes in atmospheric pressure affect the boiling point, using water as an example to illustrate that lower pressures result in lower boiling temperatures. The importance of scale accuracy when reading the graph is also emphasized.
🔍 Comparative Analysis of Vapor Pressure and Intermolecular Forces
The second paragraph delves deeper into the use of Reference Table H for comparative analysis. It highlights the ability to compare the vapor pressures of different liquids at a specific temperature, such as 60 degrees Celsius, to infer the relative strength of their intermolecular forces. The script uses the example of ethanoic acid and propanone to demonstrate that a lower vapor pressure at a given temperature indicates stronger intermolecular forces, making it harder for the liquid to turn into a gas. Furthermore, the paragraph discusses comparing the normal boiling points of different liquids at standard pressure to understand the differences in their intermolecular forces, concluding with a reminder to read the table carefully and understand the term 'normal' in the context of boiling points.
Mindmap
Keywords
💡Reference Table H
💡Vapor Pressure
💡Kinetic Energy
💡Boiling Point
💡Standard Pressure
💡Normal Boiling Point
💡Atmospheric Pressure
💡Intermolecular Forces
💡Ethanoic Acid
💡Ethanol
💡Propanone
Highlights
The video discusses Reference Table H which illustrates the relationship between temperature and vapor pressure for four different liquids.
As temperature increases, vapor pressure also increases, indicating that molecules with more kinetic energy move faster and break free as gases.
The graph in Table H shows the process of how different liquids' vapor pressures change with temperature, deemed important by people in the region.
A line is drawn at 101.3 kPa on the graph, which is the standard pressure and is related to boiling points.
The normal boiling point is the temperature at which a liquid boils at a pressure of 101.3 kPa.
Water's normal boiling point is 100 degrees Celsius at standard pressure.
Atmospheric pressure changes, such as when climbing a mountain, can affect the boiling point of a liquid.
At 70 kPa atmospheric pressure, water boils at approximately 91 degrees Celsius.
The graph in Table H allows for direct readings of boiling points at different pressures.
Comparisons can be made using Table H, such as the vapor pressures of different liquids at a specific temperature.
At 60 degrees Celsius, the order of liquids intersecting the graph indicates their relative vapor pressures and intermolecular forces.
Ethanoic acid has a low vapor pressure at 60 degrees Celsius, while propanone has a much higher vapor pressure.
The relative strength of intermolecular forces can be inferred from the vapor pressures at a particular temperature.
Table H can be used to compare the normal boiling points of different liquids at standard pressure.
Ethanol boils at a lower temperature than water at 101.3 kPa, indicating stronger intermolecular forces in water.
Propanone has the weakest intermolecular forces among the four liquids, boiling at the lowest temperature.
Table H is a valuable resource for understanding the relationship between vapor pressure, temperature, and intermolecular forces.
Careful reading of Table H is essential for accurate interpretation of data and understanding the concepts presented.
Transcripts
Browse More Related Video

2.3 Vapor Pressure, IMFs, and Boiling Point

Vapor pressure | States of matter and intermolecular forces | Chemistry | Khan Academy

Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation

Which molecules have higher (or lower) vapor pressure

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
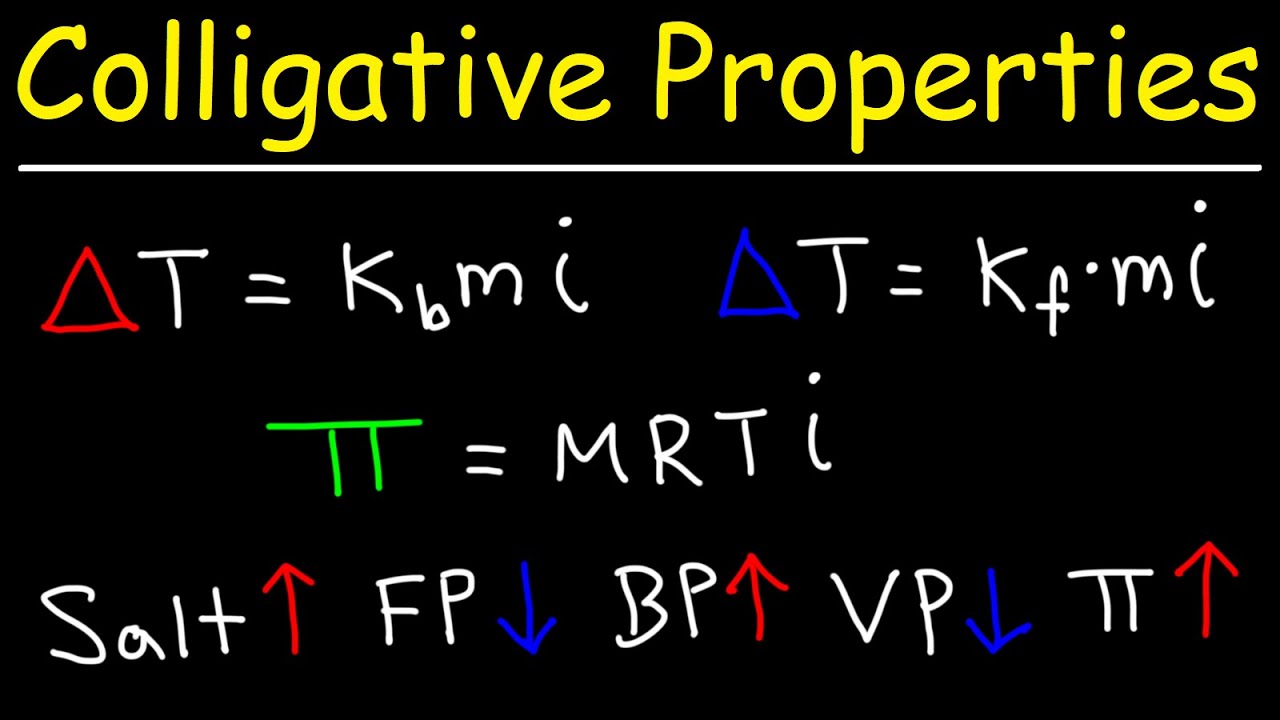
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
5.0 / 5 (0 votes)
Thanks for rating: