2.3 Vapor Pressure, IMFs, and Boiling Point
TLDRThis video explores the concept of vapor pressure and its correlation with intermolecular forces and boiling points. Using two jars with liquids at standard temperature and pressure, the presenter illustrates how the quantity of gas molecules above each liquid indicates its vapor pressure. A higher vapor pressure, as in acetone, suggests weaker intermolecular forces and a lower boiling point, whereas a lower vapor pressure, like in water, indicates stronger forces and a higher boiling point. The video encourages viewers to understand the relationship between these variables and to visualize these concepts through diagrams.
Takeaways
- 🌡️ Vapor pressure is the force exerted by a gas above a liquid, which is related to the strength of intermolecular forces.
- 🧪 Both liquids A and B are at standard temperature and pressure (STP), meaning they are at the same temperature and under the same atmospheric pressure.
- 💨 Liquid A, exemplified as acetone, has a higher vapor pressure, indicating a large amount of gas molecules above it, suggesting weaker intermolecular forces.
- 💧 Liquid B, exemplified as water, has a lower vapor pressure, indicating fewer gas molecules above it, suggesting stronger intermolecular forces.
- 🔄 The ease with which molecules can escape the liquid phase and enter the gas phase is directly related to the strength of intermolecular forces.
- 🔄 Weaker intermolecular forces make it easier for molecules to escape the liquid phase, as seen with liquid A.
- 🔗 Stronger intermolecular forces, as in liquid B, make it harder for molecules to escape, requiring more energy to transition to the gas phase.
- 🔥 The boiling point of a liquid is related to the ease of transitioning from the liquid to the gas phase; liquids with weaker intermolecular forces boil at lower temperatures.
- ❄️ Conversely, liquids with stronger intermolecular forces, like liquid B, boil at higher temperatures due to the increased energy required to overcome these forces.
- 🔄 Vapor pressure, intermolecular forces, and boiling point are interconnected variables that can be logically connected through understanding their relationships.
- 📚 It's beneficial to practice drawing diagrams to visualize the relationships between vapor pressure, intermolecular forces, and boiling points for better understanding.
Q & A
What is vapor pressure and how does it relate to the strength of intermolecular forces?
-Vapor pressure is the pressure exerted by a gas that is in equilibrium with its liquid phase. It is related to the strength of intermolecular forces because a higher vapor pressure indicates weaker intermolecular forces, making it easier for molecules to escape the liquid phase and enter the gas phase.
What does STP stand for and what are its values?
-STP stands for Standard Temperature and Pressure. Its values are 273 Kelvin or 0 degrees Celsius for temperature, and 100 kPa or 1 atmosphere for pressure.
Why does the presence of a large amount of gas above liquid A suggest a higher vapor pressure compared to liquid B?
-The presence of a large amount of gas above liquid A suggests a higher vapor pressure because it indicates that more molecules have escaped from the liquid phase to the gas phase, which implies weaker intermolecular forces in liquid A.
What is the relationship between vapor pressure and the ease of molecules escaping the liquid phase?
-Vapor pressure is directly related to the ease with which molecules can escape the liquid phase. A higher vapor pressure means that it is easier for molecules to break free from the intermolecular attractions and enter the gas phase.
How does the strength of intermolecular forces correlate with the boiling point of a substance?
-The strength of intermolecular forces is inversely correlated with the boiling point. Substances with weaker intermolecular forces have a lower boiling point because it takes less energy to transition from the liquid to the gas phase.
What can be inferred about the intermolecular forces of liquid B if it has a lower vapor pressure than liquid A?
-If liquid B has a lower vapor pressure than liquid A, it can be inferred that liquid B has stronger intermolecular forces, making it more difficult for its molecules to escape the liquid phase and enter the gas phase.
Why does the video script suggest that the molecules in liquid B are 'very attracted to one another'?
-The script suggests that the molecules in liquid B are 'very attracted to one another' because liquid B has a lower vapor pressure, indicating that its molecules have stronger intermolecular forces, making it harder for them to escape the liquid phase.
What is the significance of the 'unpleasant smell' when opening a jar containing acetone?
-The unpleasant smell when opening a jar containing acetone is due to the high vapor pressure of acetone, which results in a large number of gas molecules being released into the air, indicating weak intermolecular forces.
How can the concept of vapor pressure be used to predict the boiling point of a substance?
-Vapor pressure can be used to predict the boiling point of a substance because substances with higher vapor pressures have weaker intermolecular forces and thus require less energy to boil, leading to a lower boiling point.
What is the connection between the amount of vapor above a liquid and its vapor pressure?
-The amount of vapor above a liquid is directly related to its vapor pressure. A greater amount of vapor indicates a higher vapor pressure, which in turn suggests weaker intermolecular forces and an easier transition from the liquid to the gas phase.
Why is it important to understand the relationship between vapor pressure, intermolecular forces, and boiling point?
-Understanding this relationship is important because it helps in predicting the physical properties of substances, such as their volatility and reactivity, which are crucial in various scientific and industrial applications.
Outlines
🌡️ Vapor Pressure and Intermolecular Forces
This paragraph introduces the concept of vapor pressure in relation to the strength of intermolecular forces and boiling points of substances. The script uses the analogy of two capped jars containing liquids at standard temperature and pressure (STP), with one jar representing a liquid like acetone, which has a higher vapor pressure, and the other representing water, which has a lower vapor pressure. The higher vapor pressure of acetone is illustrated by the immediate release of gas molecules when the jar is opened, indicating a weaker intermolecular force that allows for easier escape from the liquid to the gas phase. Conversely, water's lower vapor pressure suggests stronger intermolecular forces, making it harder for molecules to transition to the gas phase.
🔥 Vapor Pressure and Boiling Points
The second paragraph delves into the connection between vapor pressure and boiling points. It explains that the ease with which a liquid can transition from a liquid to a gas phase is indicative of its boiling point. A liquid with a higher vapor pressure, like acetone, is expected to boil at a lower temperature due to the weaker intermolecular forces that require less energy to overcome. In contrast, a liquid with a lower vapor pressure, such as water, will boil at a higher temperature because of the stronger intermolecular forces that bind the molecules together more tightly. The paragraph encourages the viewer to practice visualizing these concepts and to understand the logical connections between vapor pressure, intermolecular forces, and boiling points.
Mindmap
Keywords
💡Vapor Pressure
💡Intermolecular Forces
💡Boiling Point
💡Standard Temperature and Pressure (STP)
💡Kinetic Energy
💡Acetone
💡Water
💡Molecules
💡Gas Phase
💡Liquid Phase
Highlights
The video discusses the relationship between vapor pressure and intermolecular forces or attractions.
Demonstrates the concept of vapor pressure using two capped jars containing liquids at standard temperature and pressure (STP).
Explains that vapor pressure is the force exerted by a gas above a liquid.
Hypothesizes liquid A as acetone, which has a high vapor pressure indicated by the strong smell when the jar is opened.
Contrasts liquid B as water, which has a lower vapor pressure and less noticeable gas when the jar is opened.
Relates higher vapor pressure to weaker intermolecular forces, allowing molecules to escape the liquid phase more easily.
Suggests that liquids with stronger intermolecular forces, like liquid B, are harder to evaporate and have higher boiling points.
Associates the ease of transitioning from liquid to gas with the boiling point of a substance.
Illustrates that substances with lower vapor pressure, like liquid B, require more energy to reach their boiling point.
Encourages viewers to understand the connection between vapor pressure, intermolecular forces, and boiling points.
Advises practicing by drawing diagrams to visualize the relationship between vapor pressure and intermolecular forces.
The video emphasizes the importance of recognizing the interplay between vapor pressure and the physical properties of liquids.
Uses the example of acetone and water to demonstrate the practical differences in vapor pressure.
Explains how the amount of gas above a liquid indicates the strength of intermolecular attractions.
Details the process of molecules escaping the liquid phase and the role of vapor pressure in this process.
Connects the ease of molecular escape to the strength of intermolecular forces and the substance's boiling point.
Concludes by emphasizing the importance of logical reasoning in understanding the connections between vapor pressure, intermolecular forces, and boiling points.
Transcripts
Browse More Related Video

Which molecules have higher (or lower) vapor pressure
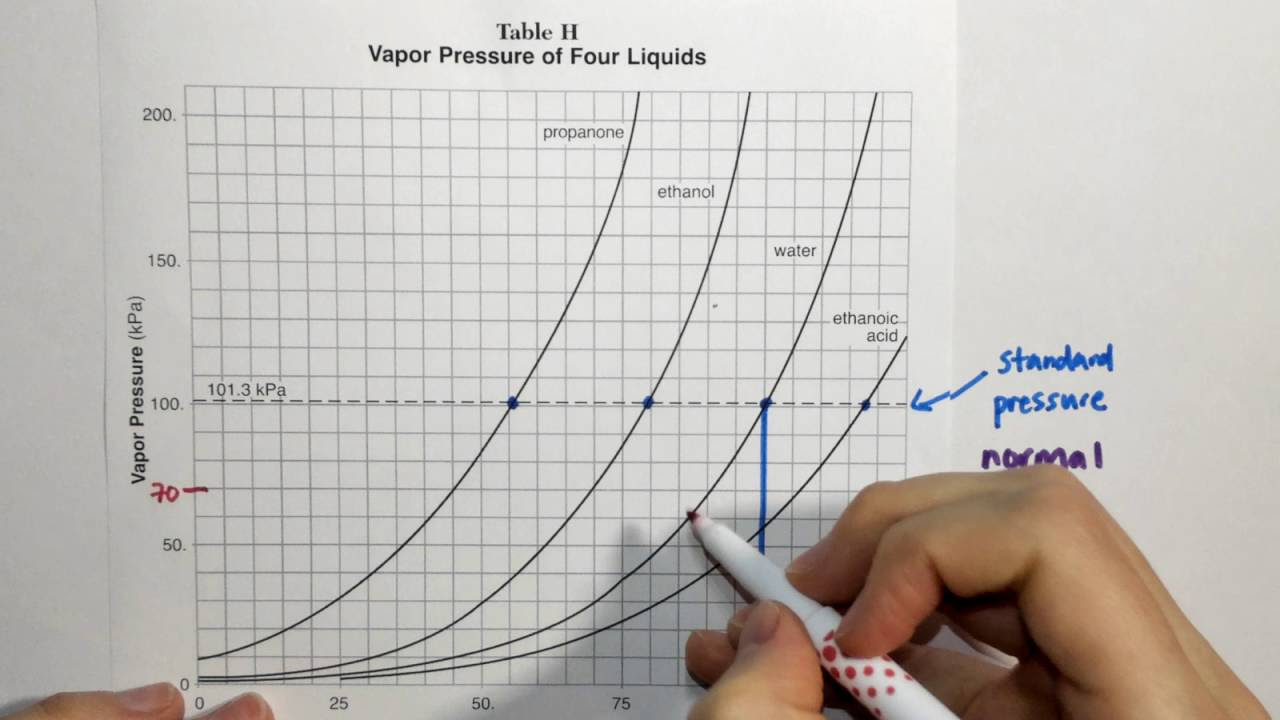
2.4 Reference Table H (Vapor Pressure and Temperature)

Vapor pressure | States of matter and intermolecular forces | Chemistry | Khan Academy

Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
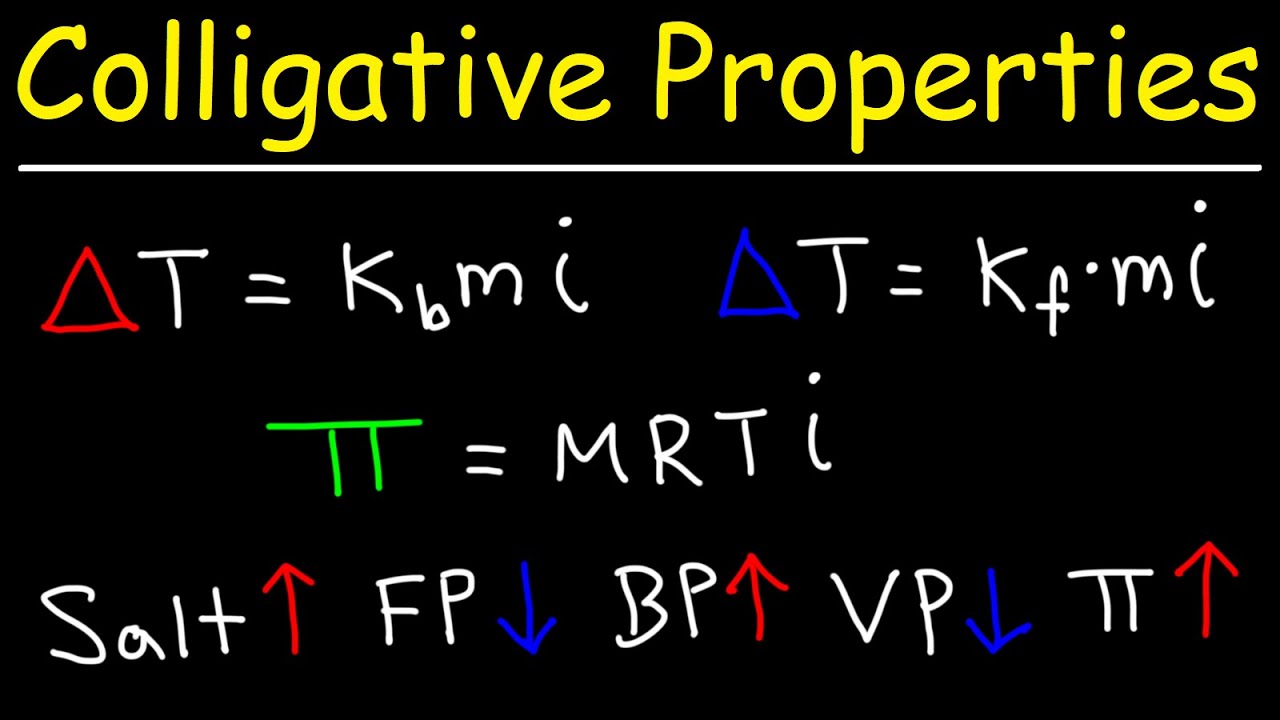
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
5.0 / 5 (0 votes)
Thanks for rating: