Vapor pressure | States of matter and intermolecular forces | Chemistry | Khan Academy
TLDRThis script delves into the concept of vapor pressure and its relationship with kinetic energy, temperature, and molecular behavior. It explains how substances in liquid form can evaporate at temperatures below their boiling point due to the distribution of kinetic energy among molecules. The video illustrates how surface molecules with sufficient energy can escape into the gaseous phase, while others return from vapor to liquid state, establishing an equilibrium. It further discusses factors affecting vapor pressure, such as molecular weight and intermolecular forces, and how it varies with different substances. The script concludes with examples of vapor pressures at various temperatures for substances like water, propane, and butane, highlighting the exponential increase in vapor pressure with temperature.
Takeaways
- 💧 In the liquid state, molecules have enough kinetic energy to move past each other but not enough to completely separate.
- 🔥 Increasing temperature and kinetic energy can overcome the heat of fusion, causing molecules to separate and form a gaseous phase.
- 🌡️ Temperature is an average measure of kinetic energy, meaning not all molecules have the same kinetic energy.
- 💨 Surface molecules are important because they are the first to vaporize if they have enough kinetic energy.
- 📊 The distribution of kinetic energy among molecules is represented by a bell curve, showing a range from low to high kinetic energy.
- 🚀 Some molecules, particularly those on the surface, can escape into the air due to having high kinetic energy.
- 🌪️ Evaporation occurs when high kinetic energy molecules escape from the liquid, even below the boiling point.
- 🔄 In a closed system, evaporation and condensation occur until an equilibrium is reached, establishing a vapor pressure.
- 🌟 Vapor pressure is the pressure created by vapor molecules at equilibrium, which varies with temperature and substance type.
- ⚖️ Vapor pressure increases exponentially with temperature, affecting the volatility and phase state of substances.
- 🌍 The boiling point of a substance is reached when its vapor pressure equals the atmospheric pressure.
Q & A
What is the kinetic energy of molecules in a liquid state?
-In a liquid state, molecules have enough kinetic energy to move past each other, allowing for flow, but not enough to completely move away from each other, maintaining some bonds and staying close.
How does increasing temperature affect the phase of a substance?
-Increasing the temperature, and thus the average kinetic energy, can overcome the heat of fusion, causing the substance to transition from a liquid to a gaseous phase where molecules have even more kinetic energy and move freely.
What is the significance of temperature in terms of kinetic energy?
-Temperature is a measure of the average kinetic energy of the molecules in a substance, indicating that not all molecules have the same kinetic energy and there is a distribution of energies.
Why are surface molecules important in the context of phase changes?
-Surface molecules are important because they are the first ones capable of leaving the liquid and transitioning into the gaseous phase if they have enough kinetic energy.
What is the process called when molecules escape from a liquid into a gas?
-The process is called evaporation, where molecules with unusually high kinetic energy escape from the liquid surface and enter the gaseous state.
What happens to the molecules that escape during evaporation?
-The escaped molecules can be carried away by factors such as wind in an open system, or in a closed system, they may mix with the gas molecules above the liquid.
What is vapor pressure and how is it related to evaporation?
-Vapor pressure is the pressure created by the vapor molecules above a liquid at a given temperature where evaporation and condensation are in equilibrium. It is directly related to the tendency of a substance to evaporate.
How does the volatility of a substance relate to its vapor pressure?
-A substance with high volatility, such as gasoline, has a higher vapor pressure because more of its molecules enter the vapor state at a given temperature, creating more pressure to offset its natural inclination to evaporate.
At what point does a liquid start to boil?
-A liquid starts to boil when its vapor pressure becomes equal to the atmospheric pressure, allowing the vapor to push against the atmosphere and the liquid to transition into the gaseous state.
How does lowering the atmospheric pressure affect the boiling point of a substance?
-Lowering the atmospheric pressure reduces the external pressure on the liquid, causing the substance to boil at a lower temperature because the vapor pressure can more easily equalize with the reduced atmospheric pressure.
What factors influence the vapor pressure of a substance?
-Factors that influence vapor pressure include the temperature, the intermolecular forces, and the molecular mass of the substance. Higher temperatures, lower intermolecular forces, and lighter molecules generally result in higher vapor pressures.
Outlines
🌡 Understanding Liquid States and Evaporation
This paragraph explains the concept of a substance in a liquid state, where molecules have enough kinetic energy to move past each other but not enough to separate completely. It describes how increasing the temperature can lead to the molecules overcoming the heat of fusion and transitioning into a gaseous phase. The paragraph also touches on the idea that temperature represents average kinetic energy, meaning not all molecules have the same energy levels. It introduces the concept of surface molecules having the potential to vaporize due to their higher kinetic energy and the role of evaporation in this process, even at temperatures below the boiling point.
🔄 The Dynamics of Evaporation and Vapor Pressure
The second paragraph delves into the process of evaporation within a closed system and the concept of vapor pressure. It discusses how in a closed system, evaporation and condensation occur simultaneously, leading to an equilibrium state where the rate of molecules escaping into the vapor phase is balanced by the rate of vapor molecules returning to the liquid phase. The paragraph explains that vapor pressure is the pressure exerted by vapor molecules at equilibrium and varies depending on the substance and temperature. It also explores factors that influence vapor pressure, such as molecular weight, intermolecular forces, and volatility.
📈 Vapor Pressure and the Boiling Point
This paragraph focuses on the relationship between vapor pressure and the boiling point of a substance. It illustrates how a substance reaches its boiling point when its vapor pressure equals the external atmospheric pressure. The explanation includes how substances with high vapor pressure, such as gasoline, are more volatile and will evaporate more readily compared to substances with low vapor pressure, like water. The paragraph also discusses how the boiling point can be influenced by changes in atmospheric pressure, leading to boiling at lower temperatures when the pressure is reduced.
📊 Comparing Vapor Pressures and Volatility
The final paragraph provides a comparative analysis of vapor pressures of different substances at various temperatures. It uses charts to demonstrate the exponential increase in vapor pressure with temperature and how this affects the volatility of substances. The paragraph highlights examples such as propane, which has a high vapor pressure and boils at 20 degrees Celsius, and butane, which remains liquid at room temperature under increased pressure. It concludes by emphasizing the practical implications of understanding vapor pressure in everyday scenarios.
Mindmap
Keywords
💡Kinetic Energy
💡Molecules
💡Liquid State
💡Gaseous Phase
💡Temperature
💡Heat of Fusion
💡Vapor Pressure
💡Evaporation
💡Boiling Point
💡Intermolecular Forces
💡Volatility
Highlights
Liquid substances have molecules with enough kinetic energy to move past each other but not enough to completely separate.
Molecules in a liquid are held together by bonds that allow them to switch between different molecules but remain close.
Increasing the temperature can overcome the heat of fusion, causing molecules to separate and enter a gaseous phase.
Temperature is a measure of average kinetic energy, implying a distribution of kinetic energies among molecules.
Surface molecules are the first to vaporize due to their direct interaction with the external pressure.
A distribution graph of kinetic energy for surface molecules shows a range from low to high kinetic energy.
Some molecules have enough kinetic energy to escape the liquid and become gas, a process known as evaporation.
Evaporation occurs even below the boiling point due to molecules with unusually high kinetic energy escaping.
In a closed system, evaporation and condensation occur simultaneously until an equilibrium is reached.
Vapor pressure is the pressure created by vapor molecules at equilibrium with the liquid.
Vapor pressure varies with temperature and is different for each substance.
Molecules with high kinetic energy, low intermolecular forces, or light mass have a higher tendency to evaporate.
High vapor pressure substances reach equilibrium with more molecules in the vapor state.
Substances with low vapor pressure, strong intermolecular forces, or heavy mass are less likely to evaporate.
Vapor pressure equals atmospheric pressure at the boiling point, allowing molecules to escape and form a gas.
Vapor pressure of water at 100 degrees Celsius is 760 torr, which is equal to atmospheric pressure.
Lowering atmospheric pressure allows substances to boil at lower temperatures due to reaching their vapor pressure.
Vapor pressure increases exponentially with temperature, affecting the rate of evaporation.
Different substances have different vapor pressures at the same temperature, affecting their volatility.
Propane has a high vapor pressure and boils at 20 degrees Celsius under atmospheric pressure.
Adjusting pressure can control the state of a substance, such as keeping butane in a liquid state in lighters.
Transcripts
Browse More Related Video
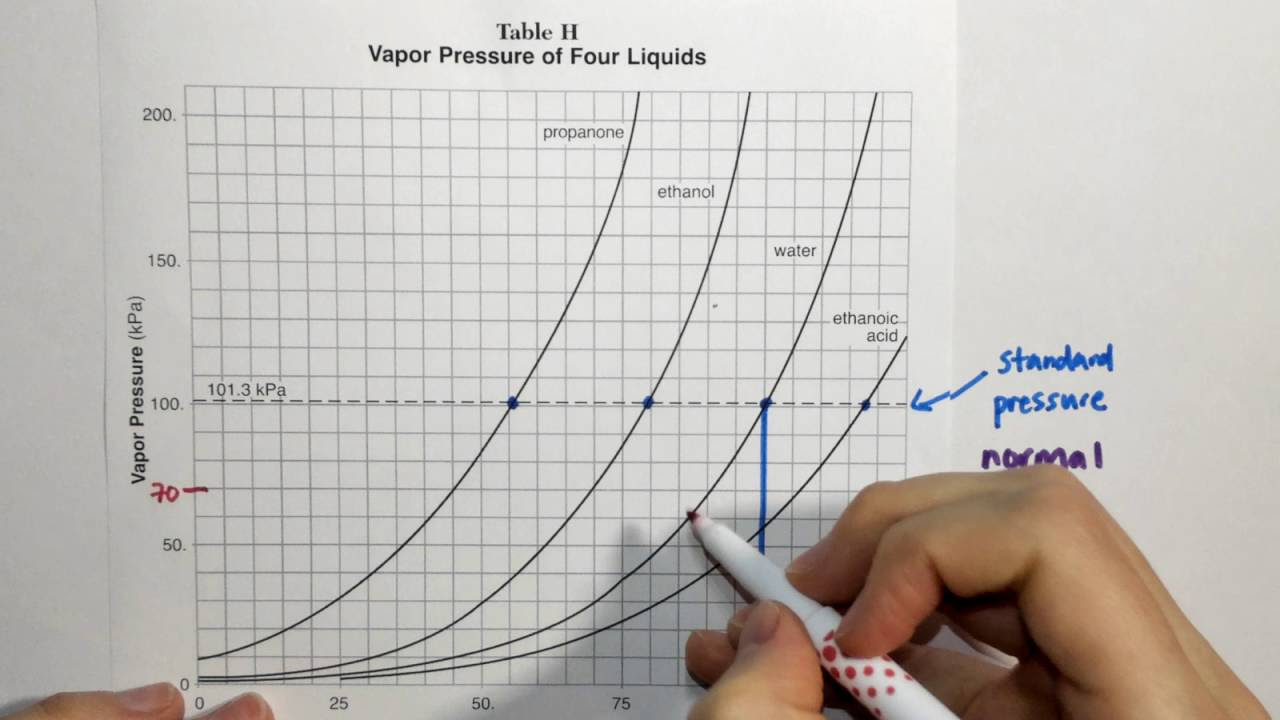
2.4 Reference Table H (Vapor Pressure and Temperature)

2.3 Vapor Pressure, IMFs, and Boiling Point

reading water tables

Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation

Which molecules have higher (or lower) vapor pressure

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
5.0 / 5 (0 votes)
Thanks for rating: