Organic Chemistry Introduction Part 2
TLDRThis video script introduces various functional groups in organic chemistry, explaining their structures and examples. It highlights common mistakes, such as confusing alcohols with carboxylic acids or aldehydes with ketones, and emphasizes the importance of checking the connections of functional groups. Resources for further help are also mentioned.
Takeaways
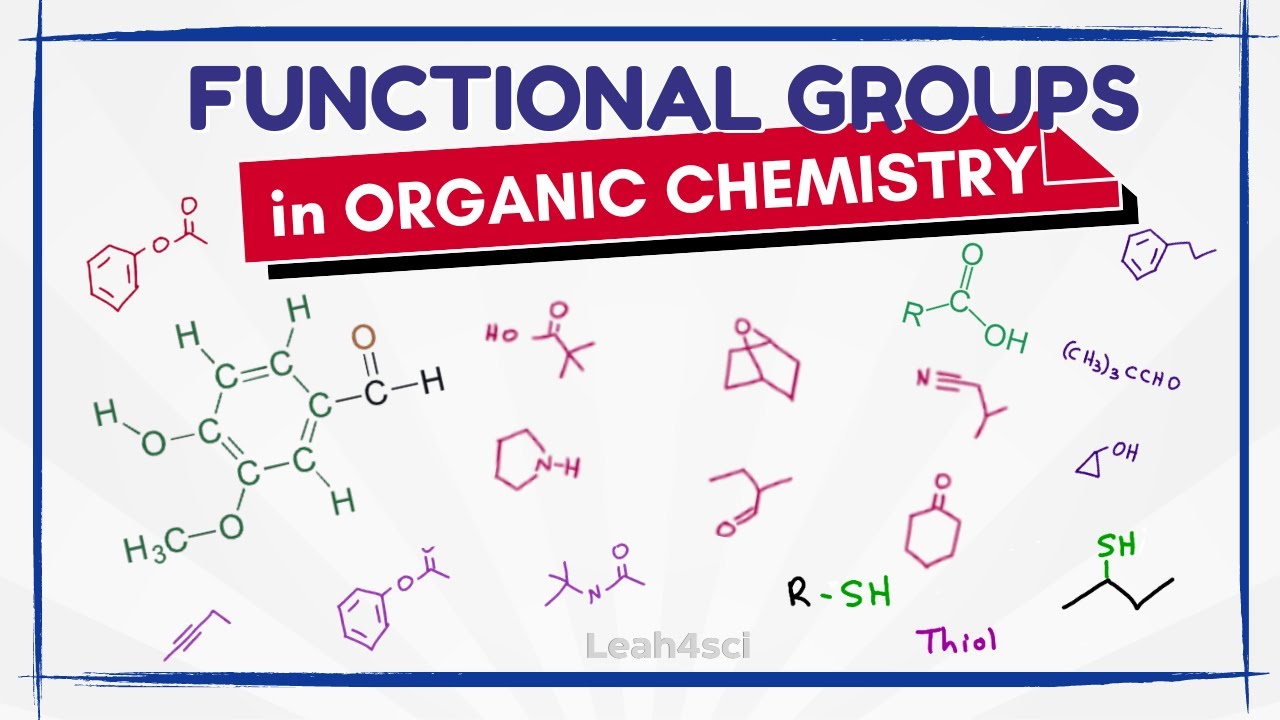
- 🧪 Functional groups are specific types of substituents attached to a carbon in a carbon chain.
- 🔍 An R group represents the rest of the molecule, which can be a carbon chain or other structures.
- 🔗 Alkenes are characterized by a double bond, while alkynes have a triple bond.
- 🌐 Alkyl halides consist of carbon and hydrogen with a halogen attached.
- 🍺 Alcohols have an OH group bonded to a carbon chain or R group.
- 🌀 Ethers feature an oxygen atom surrounded by two R groups, which can be the same or different.
- 🔶 Epoxides have a triangular shape with oxygen at the top of the triangle.
- 🍂 Ketones have a carbon double-bonded to oxygen and attached to two R groups.
- 🍂 Aldehydes are similar to ketones but have a hydrogen instead of two R groups.
- 🍋 Carboxylic acids have a carbon double-bonded to oxygen with an OH group instead of an R group or hydrogen.
- 🍇 Esters have a structure similar to carboxylic acids but with an R group attached to the oxygen.
- 🍃 Amides have a nitrogen attached to the carbon that is double-bonded to oxygen.
- 🌸 Amines contain nitrogen but do not have a carbon double-bonded to an oxygen.
- 🌹 A phenyl group is a benzene ring attached to an R group, and aromatic describes a benzene ring.
- 🚫 Common mistake: Students often confuse alcohol groups with carboxylic acids; always check the connection of the OH group.
- 🚫 Another common mistake is confusing aldehydes with ketones; check the number of R groups attached to the carbon double-bonded to oxygen.
Q & A
What is a functional group in organic chemistry?
-A functional group is a specific type of substituent that is still attached to a carbon in the carbon chain. It determines the chemical properties and reactivity of the molecule.
What does the term 'R group' represent in organic chemistry?
-The term 'R group' stands for the rest of the molecule, indicating the part of the molecule that is not part of the functional group.
What is the structural formula for an alkene?
-An alkene is characterized by a carbon-carbon double bond in its structural formula.
How does an alkyne differ from an alkene in terms of bonding?
-An alkyne has a carbon-carbon triple bond, which is different from the double bond found in alkenes.
What is an alkyl halide and what does it consist of?
-An alkyl halide consists of carbon and hydrogen (alkyl) and a halogen. It is a type of functional group where a halogen atom is attached to an alkyl group.
What is the key characteristic of an alcohol in organic chemistry?
-An alcohol is characterized by an OH (hydroxyl) group bonded to a carbon chain or R group.
How is an ether different from an alcohol in terms of its structure?
-An ether has an oxygen atom in the center, surrounded by two R groups, whereas an alcohol has an OH group bonded to a carbon chain.
What is the structural difference between a ketone and an aldehyde?
-A ketone has a carbon double-bonded to oxygen that is attached to two R groups, while an aldehyde has a carbon double-bonded to oxygen with one hydrogen atom and one R group.
How can you distinguish a carboxylic acid from an alcohol based on their structures?
-A carboxylic acid has an OH group attached to a carbon that is double-bonded to oxygen, whereas an alcohol has an OH group bonded to a carbon chain or R group.
What is an ester and how does its structure differ from that of a carboxylic acid?
-An ester has a similar structure to a carboxylic acid, but instead of an OH group, it has an R group attached to the oxygen.
What is the key difference between an amide and an amine in terms of their structures?
-An amide has a nitrogen atom attached to the carbon that is double-bonded to oxygen, while an amine has nitrogen but does not have a carbon double-bonded to an oxygen.
What is a common mistake students make when identifying functional groups, and how can it be avoided?
-A common mistake is confusing a carboxylic acid for an alcohol or an aldehyde for a ketone. Students should always check what the OH or carbonyl group is connected to and ensure they understand the difference between these functional groups.
Outlines
🌟 Introduction to Functional Groups
This paragraph introduces the concept of functional groups in organic chemistry, emphasizing their role as specific types of substituents attached to a carbon in a carbon chain. The explanation begins by defining an R group as the 'rest of the molecule' and provides an example of a functional group with a carbon chain and a halogen. The paragraph then lists and describes the most common functional groups, including alkenes, alkynes, alkyl halides, alcohols, ethers, epoxides, ketones, aldehydes, carboxylic acids, esters, amides, amines, and phenyl groups. The importance of understanding the structure and naming of these groups is highlighted, with a focus on avoiding common mistakes in their identification.
Mindmap
Keywords
💡Functional Group
💡R Group
💡Alkene
💡Alkyne
💡Alkyl Halide
💡Alcohol
💡Ether
💡Epoxide
💡Ketone
💡Aldehyde
💡Carboxylic Acid
💡Ester
💡Amide
💡Phenyl Group
💡Aromatic
Highlights
Introduction to functional groups in organic chemistry.
Functional groups are specific types of substituents attached to a carbon in the carbon chain.
Definition of an R group as the rest of the molecule.
Example of a functional group with a generic structural formula.
Explanation of the alkene functional group referring to a double bond.
Description of the alkyne functional group indicating a triple bond.
Introduction to the alkyl halide functional group consisting of carbon, hydrogen, and a halogen.
Alcohol functional group characterized by an OH bonded to a carbon chain or R group.
Ether functional group with an oxygen in the center surrounded by two R groups.
Epoxide functional group with a triangular shape and oxygen at the top.
Ketone functional group with a carbon double-bonded to oxygen and attached to two R groups.
Aldehyde functional group similar to a ketone but with a hydrogen instead of two R groups.
Carboxylic acid functional group with a carbon double-bonded to oxygen and an OH instead of an R group or hydrogen.
Ester functional group with a similar structure to a carboxylic acid but with an R group attached to the oxygen.
Amide functional group with a nitrogen attached to the carbon double-bonded to oxygen.
Ammine functional group with nitrogen but without a carbon double-bonded to an oxygen.
Phenyl group described as a benzene ring attached to an R group.
Aromatic functional group name for a benzene ring.
Potential quiz or test question involving identifying functional groups in a molecule.
Common mistake of mistaking a carboxylic acid for an alcohol group.
Confusion between an aldehyde and a ketone, explained by the number of R groups.
Recommendation for additional resources in the description box for further help.
Encouragement to stay determined in learning organic chemistry.
Transcripts
Browse More Related Video

Identifying functional groups | Organic chemistry | Khan Academy
Functional Groups with Memorization Tips

identifying organic functional groups

Hydrocarbon Derivatives: Crash Course Chemistry #43

More Organic Nomenclature: Heteroatom Functional Groups: Crash Course Organic Chemistry #3

Identifying Functional Groups | Study With Us
5.0 / 5 (0 votes)
Thanks for rating: