A Love Letter to H2O: Water & pH: Crash Course Biology #21
TLDRThis script from Crash Course Biology delves into the history and science of water, exploring its molecular structure, polarity, and vital role in sustaining life. It recounts the discovery of hydrogen and oxygen by Lavoisier and Cavendish, and explains water's unique properties, such as its polarity leading to its exceptional solvent capabilities, its behavior as a coolant through evaporation, and its significance in the pH scale. The script emphasizes water's irreplaceability and its importance in biological and environmental processes, highlighting its romanticized status as the hero of the story.
Takeaways
- 🔬 Antoine-Laurent Lavoisier is known as the father of modern chemistry and was the first to name the gas oxygen.
- 🔬 Henry Cavendish discovered hydrogen while burning an unnamed gas in an oxygen-rich environment, which resulted in the formation of water.
- 💧 Lavoisier demonstrated that water is composed of hydrogen and oxygen, a discovery crucial to understanding its life-sustaining properties.
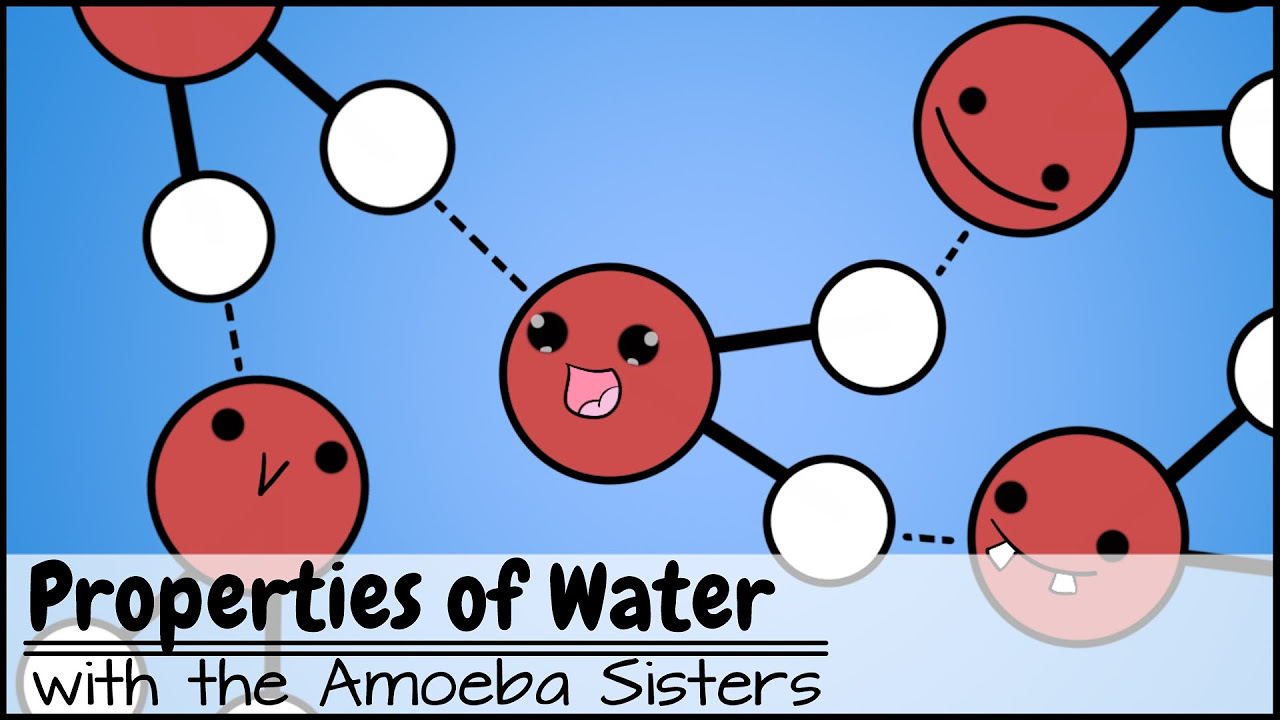
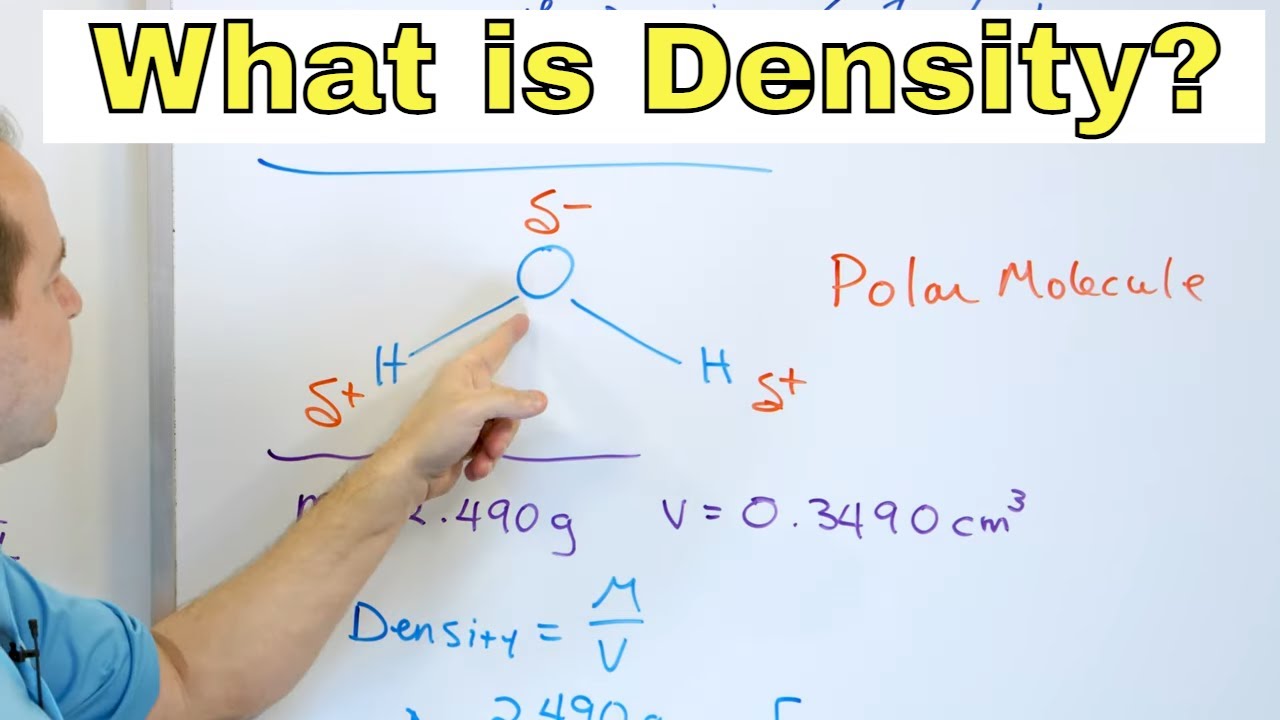
- 🌐 Water's polarity, due to the covalent bond between hydrogen and oxygen, is fundamental to its unique characteristics and its role in sustaining life.
- 🧲 Water acts as a universal solvent, dissolving many substances like sugar and salt, which is vital for cellular processes and metabolism.
- 🚫 Water's polarity also prevents nonpolar substances like fats from dissolving, which is important for maintaining cellular structures.
- 🧊 The properties of ice, such as its lower density allowing it to float, are due to the hydrogen bonds that form a more open structure compared to liquid water.
- 🌡 Water's surface tension, a result of hydrogen bonding, allows creatures like water striders to walk on its surface and helps in the transport of water in plants.
- 💦 Evaporation of water helps regulate body temperature in organisms and plays a role in the water cycle, buffering temperature changes on Earth.
- ⚗️ The pH scale measures the acidity or basicity of aqueous solutions, with water being neutral at pH 7, indicating its importance in maintaining chemical balance in biological systems.
- 🌍 Scientists search for water on other planets as an indicator of life, highlighting water's irreplaceable role in the existence of life as we know it.
Q & A
Who is Antoine-Laurent Lavoisier and why is he significant in the history of chemistry?
-Antoine-Laurent Lavoisier is a French chemist often referred to as the father of modern chemistry. He is significant for his work in identifying and naming the element oxygen and for his role in demonstrating the composition of water as a combination of hydrogen and oxygen.
What role did Henry Cavendish play in the discovery of hydrogen?
-Henry Cavendish, an English chemist, was conducting experiments with an unnamed gas in an oxygen-rich environment, which resulted in the production of water. Lavoisier later replicated Cavendish's experiment using an electrical spark and named the gas hydrogen.
How did Lavoisier demonstrate that water is composed of hydrogen and oxygen?
-Lavoisier demonstrated the composition of water by replicating Cavendish's experiment but using an electrical spark. Through this process, he was able to show that water is made up of hydrogen and oxygen.
What is the significance of water's polarity and how does it affect its properties?
-Water's polarity, due to the slight negative charge on the oxygen atom and the slight positive charge on the hydrogen atoms, gives it unique properties such as being a universal solvent, having high surface tension, and forming hydrogen bonds, which are essential for life.
Why is water considered a universal solvent?
-Water is considered a universal solvent because of its polarity, which allows it to dissolve many substances such as salts and sugars. The polar nature of water enables it to surround and separate ions, facilitating the dissolution process.
What is the concept of surface tension in water and why is it important?
-Surface tension in water is the result of cohesion, the force that causes water molecules to stick to each other due to hydrogen bonding. This property is important as it allows certain insects, like water striders, to walk on water's surface and plays a role in the transport of water in plants.
How does the structure of ice differ from liquid water and why does ice float?
-In ice, water molecules form more hydrogen bonds, which causes them to arrange in a more open, crystalline structure. This makes ice less dense than liquid water, allowing it to float, which is unusual for solids.
What is the pH scale and how does water relate to it?
-The pH scale measures the acidity or basicity of an aqueous solution. Water has a neutral pH of 7, meaning it is neither acidic nor basic. It can form both hydrogen ions (H+) and hydroxide ions (OH-), contributing to the balance of pH in solutions.
Why is water essential for life on Earth and what would happen if it were replaced by another substance like ethanol?
-Water is essential for life due to its unique properties, such as polarity, solvent capabilities, and ability to buffer temperature changes. If water were replaced by ethanol, which forms fewer hydrogen bonds, it would evaporate more quickly, leading to a loss of liquid water and making it difficult for life as we know it to exist.
What is the significance of water's role in the evaporation process and the water cycle?
-Water's role in evaporation and the water cycle is crucial for temperature regulation on Earth. Evaporation from bodies of water helps to cool the environment, while the water cycle ensures the distribution of water, replenishing water sources and supporting life.
How does water's ability to buffer drastic temperature changes contribute to the stability of Earth's climate?
-Water's high specific heat capacity allows it to absorb and release large amounts of heat with minimal temperature change. This property helps to moderate temperature fluctuations, preventing extreme increases during the day and extreme decreases at night.
Outlines
🔬 Discovering the Elements of Water
This paragraph delves into the historical discovery and understanding of water's composition. Antoine-Laurent Lavoisier, known as the father of modern chemistry, named the previously unknown gas oxygen after replicating an experiment. Concurrently, Henry Cavendish, an English chemist, identified water as a product of burning another unnamed gas in oxygen-rich conditions, later named hydrogen by Lavoisier. Lavoisier's further experiments demonstrated that water is composed of hydrogen and oxygen. The paragraph highlights the significance of these discoveries for our current knowledge of water's life-sustaining properties and its role in various applications, including its unfortunate use in blimps like the Hindenburg.
💧 The Science of Water's Polarity and Solvent Properties
The second paragraph explores the chemical composition of water, emphasizing its polarity due to the covalent bond between hydrogen and oxygen atoms. This polarity results in water's unique V-shaped molecular structure and its ability to act as a universal solvent, dissolving many substances like sugar and salt. The paragraph also explains how water's polarity influences its interactions with other molecules, such as lipids and ionic salts, which is crucial for cellular processes and metabolic reactions in living organisms.
❄️ Ice, Water's Unique Solid State and Other Properties
This paragraph examines the peculiar properties of ice, water's solid state, including its ability to float due to its lower density compared to liquid water, a result of the expanded structure from increased hydrogen bonding. It also touches on water's surface tension, which allows insects like water striders to walk on water's surface. The discussion extends to water's role in temperature regulation through evaporation and its importance in the water cycle, which helps buffer temperature extremes on Earth. The paragraph concludes with a hypothetical scenario comparing water to ethanol, illustrating the critical role water plays in supporting life.
🌡️ Water's Role in pH Balance and Its Universal Significance
The final paragraph discusses water's neutrality on the pH scale, which is vital for maintaining the balance of chemical reactions in living organisms, including the human body. It explains how water can dissociate into hydrogen and hydroxide ions, affecting the pH levels in solutions. The paragraph also emphasizes water's importance as a universal solvent and its role in the transport and regulation of substances within organisms. It concludes by recognizing water's significance in the search for extraterrestrial life and its integral part in the production of the educational series, Crash Course Biology.
Mindmap
Keywords
💡Antoine-Laurent Lavoisier
💡Henry Cavendish
💡Hydrogen
💡Oxygen
💡Covalent Bond
💡Polar Molecule
💡Solvent
💡Surface Tension
💡Evaporation
💡pH Scale
💡Chemical Buffers
Highlights
Studying gases was a significant scientific pursuit in the 18th century, with Lavoisier naming 'oxygen' and 'hydrogen'.
Lavoisier and Cavendish's experiments demonstrated that water is composed of hydrogen and oxygen.
Water's polar nature, due to its molecular structure, is crucial for its life-sustaining properties.
Water's polarity allows it to act as a universal solvent, dissolving many substances.
The molecular structure of water leads to its unique property of repelling nonpolar substances like lipids.
Water's ability to dissolve ionic compounds is vital for chemical reactions and metabolism in organisms.
Hydrogen bonding in water molecules results in cohesion, which is evident in water's surface tension.
Ice's lower density compared to liquid water is due to the hydrogen bonds that cause it to be less dense and float.
Water's role in the water cycle helps regulate Earth's temperature by buffering against extreme temperature changes.
The pH scale is used to measure the acidity or basicity of aqueous solutions, with water being neutral at pH 7.
Water's neutrality and ability to buffer pH changes are essential for maintaining life's delicate balance.
Ethanol, as a substitute for water, would drastically affect life due to its lower hydrogen bonding and surface tension.
Water's unique properties make it a key indicator for the search of life on other planets.
The importance of water is highlighted by its presence in every aspect of life, from the ocean to a cup of tea.
The episode concludes with a poetic tribute to water, emphasizing its irreplaceable role in the universe.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: