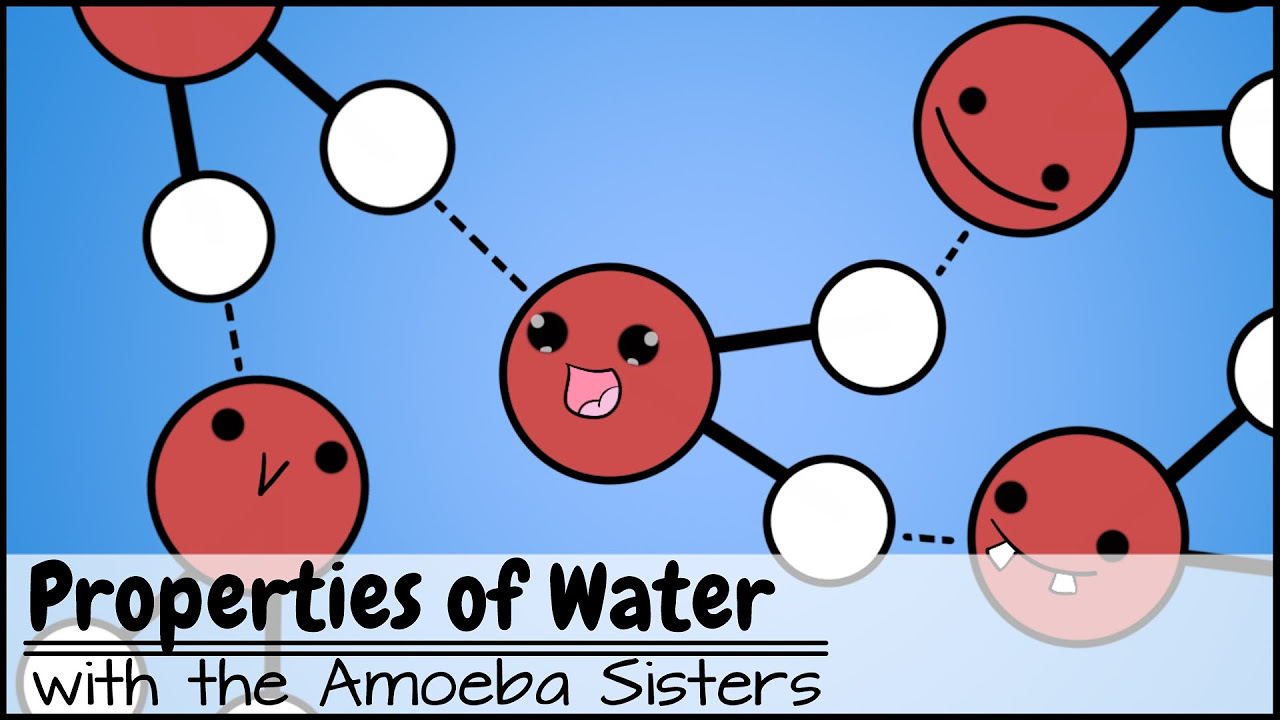
How polarity makes water behave strangely - Christina Kleinberg
TLDRThe video script explains the unique properties of water due to its polar molecule structure, which leads to polar covalent bonds and hydrogen bonding. These characteristics give rise to water's cohesion and adhesion, allowing insects to walk on its surface and causing ice to float, which is essential for aquatic life during winter. The polarity of water is fundamental to its life-sustaining role in various environments.
Takeaways
- 💧 Water is a polar molecule with unique properties due to the unequal sharing of electrons between oxygen and hydrogen atoms.
- 🔬 Oxygen atoms in water have a stronger attraction to electrons, resulting in a negative charge, while hydrogen atoms have a positive charge.
- 🤝 The polarity of water molecules leads to the formation of hydrogen bonds, which are a special type of intermolecular bond.
- 🧲 Hydrogen bonds cause water to have high surface tension, allowing some insects to 'walk on water' while humans cannot due to their weight.
- ⛄️ Ice is less dense than liquid water because the hydrogen bonds in ice keep the molecules farther apart, causing it to float on the surface.
- ❄️ Lakes freeze from the top down in winter because ice, being less dense, floats, providing a protective layer for aquatic life underneath.
- 🧲 Cohesion is water's ability to stick to itself, while adhesion is its ability to stick to other substances, both facilitated by hydrogen bonding.
- 🌡️ Water's polarity contributes to its high heat capacity, allowing it to absorb and release a significant amount of heat without a large change in temperature.
- 🌿 Water's unique properties are essential to life, as it makes up a large percentage of the human body and is crucial for various biological processes.
- 🌎 The polarity of water molecules is responsible for its role in the Earth's climate system, including weather patterns and the water cycle.
- 🧬 At the cellular level, water's polarity is vital for the structure and function of cells, as it helps to maintain cell shape and facilitates biochemical reactions.
Q & A
What is the primary reason insects can walk on the surface of a pond without sinking?
-Insects can walk on water due to the surface tension created by hydrogen bonding, which forms a thin film on the water's surface that provides enough resistance for super-light insects to walk on.
Why do humans and other heavier objects sink when attempting to walk on water?
-Humans and other heavy objects sink because the hydrogen bonds at the water's surface are not strong enough to support their weight.
How does the polarity of water molecules contribute to water's unique properties?
-The polarity within water molecules, which results from the unequal sharing of electrons between oxygen and hydrogen atoms, leads to the formation of polar covalent bonds and hydrogen bonds, giving water its unique properties like cohesion and adhesion.
Why does ice float on top of liquid water, unlike most other substances?
-Ice floats on water because the hydrogen bonds in frozen water keep the molecules farther apart, making the solid state of water (ice) less dense than its liquid state.
What percentage is ice less dense than liquid water, and why is this significant?
-Ice is about 9% less dense than water, which allows it to float on the surface. This is significant because it enables aquatic life to survive under the ice during cold winters.
How does the polarity of water molecules affect the human body?
-The polarity of water molecules is essential for life as water makes up approximately 60% of the adult human body weight, playing a critical role in various biological processes.
What is cohesion in the context of water?
-Cohesion refers to water's ability to stick to itself due to hydrogen bonding between water molecules.
What is adhesion in relation to water and other substances?
-Adhesion is the ability of water to stick to other substances, which is also a result of hydrogen bonding between water molecules and polar or ionic substances.
Why does water have a higher boiling point than expected for a molecule of its size?
-Water has a higher boiling point due to the strong hydrogen bonding between its molecules, which requires more energy to break.
How do the properties of water contribute to its role in supporting life?
-The unique properties of water, such as its polarity, hydrogen bonding, cohesion, and adhesion, allow it to act as a universal solvent and participate in biochemical reactions, which are essential for life.
What is the significance of water's polarity in terms of its reactivity with other substances?
-The polarity of water allows it to dissolve many substances, facilitating chemical reactions and nutrient transport, which are vital for living organisms.
Why does water freeze from the top down, and what impact does this have on aquatic life?
-Water freezes from the top down because ice, being less dense, floats. This process creates an insulating layer of ice that protects aquatic life from the cold temperatures, allowing them to survive the winter.
Outlines
🌊 Water Polarity and its Unique Properties
The first paragraph introduces the concept of polarity in water molecules and its significance to life. Water is a polar molecule, made up of one oxygen atom and two hydrogen atoms, which gives it unique properties. The oxygen atom attracts more electrons due to its larger size and greater number of protons, creating a negative charge, while the hydrogen atoms, being smaller, attract fewer electrons and have a positive charge. This polarity leads to the formation of polar covalent bonds and hydrogen bonds between water molecules, resulting in cohesion (water sticking to itself) and adhesion (water sticking to other substances). The paragraph also addresses why some insects can walk on water due to surface tension from hydrogen bonding, and why ice floats on water because the hydrogen bonds in ice keep the molecules farther apart, making it less dense and therefore less heavy than liquid water.
Mindmap
Keywords
💡Polarity
💡Water Molecule
💡Hydrogen Bond
💡Surface Tension
💡Cohesion
💡Adhesion
💡Density
💡Freezing
💡Aquatic Life
💡Oxygen Atom
💡Hydrogen Atom
Highlights
Water's polarity gives it unique and life-sustaining properties.
Polarity refers to the unequal sharing of electrons within a molecule.
Oxygen attracts more electrons due to its larger size and more protons.
Hydrogen, being smaller, attracts fewer electrons in a water molecule.
Water molecules form polar covalent bonds, where electrons are shared unequally.
Hydrogen bonds form between water molecules, contributing to water's cohesion and adhesion.
Insects can walk on water due to surface tension created by hydrogen bonding.
Ice floats on water because the hydrogen bonds make it less dense than liquid water.
Lakes freeze from the top down, allowing aquatic life to survive through winter.
The polarity of water molecules and resulting hydrogen bonding explain water's special properties.
Water is essential to life, making up approximately 60% of an adult human's body weight.
The oxygen in water behaves as though it's negative, while the hydrogens behave as though they're positive.
Water's ability to stick to itself is called cohesion, and to other substances is called adhesion.
Most substances are denser in solid state, but water is an exception due to hydrogen bonding.
The reason water is special, from cellular to oceanic levels, is because it is a polar molecule.
The unique properties of water are due to its molecular structure and hydrogen bonding.
Hydrogen bonds are not exclusive to water and can form with other polar or ionic substances.
The strength of hydrogen bonds is insufficient to support the weight of humans on water's surface.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: