Water Chemistry
TLDRThe video script delves into the fundamental chemistry of water, highlighting its polar nature due to the unequal sharing of electrons between oxygen and hydrogen atoms. This polarity leads to hydrogen bonding, which is crucial for water's unique properties such as high specific heat, cohesion, and adhesion. The high specific heat allows water to absorb a significant amount of heat before reaching boiling point, which is essential for maintaining a stable body temperature in living organisms. Cohesion, the attraction between water molecules, and adhesion, the attraction between water and other substances, are demonstrated through examples like a paper clip floating on water and the meniscus in a graduated cylinder. The script also explores the concept of solutions, distinguishing between solutes and solvents, and explains the importance of pH levels in biological solutions like blood, which naturally has a slightly basic pH of around 7.4. It warns of the dangers of alcohol abuse, which can disrupt this pH balance and lead to severe health consequences.
Takeaways
- 🌊 Water is a polar molecule with a slightly positive hydrogen side and a slightly negative oxygen side due to the unequal sharing of electrons.
- 🔬 Oxygen atoms in water 'hog' electrons from hydrogen atoms, resulting in a molecule with a negative charge on the oxygen side and a positive charge on the hydrogen side.
- 🤝 The polarity of water leads to hydrogen bonding, where one water molecule's hydrogen end is attracted to another water molecule's oxygen end, forming a bond.
- 🌡 Water has a high specific heat capacity, meaning it can absorb a lot of heat before it starts to boil or cool down significantly, which helps maintain a stable body temperature in organisms.
- 🏋️♂️ Physical activity generates heat, but the high specific heat of water in our bodies prevents rapid temperature changes, as demonstrated by athletes whose body temperatures remain relatively stable.
- 💧 Cohesion is the property where water molecules stick to each other due to hydrogen bonding, allowing light objects like paper clips to float on water's surface.
- 🧲 Adhesion is the property where water sticks to other substances, such as the glass of a graduated cylinder, causing a meniscus or dip in the water level.
- 🍹 Solutions are mixtures where a solute (the substance being dissolved) is mixed with a solvent (the substance doing the dissolving, often water).
- 🩸 Blood is an example of a biological solution, with plasma as the solvent and various solutes like carbohydrates, proteins, and sugars dissolved within it.
- 📊 The pH scale measures the acidity or alkalinity of a solution, with 7 being neutral, below 7 being acidic (with more hydrogen ions), and above 7 being basic (with more hydroxide ions).
- 🍋 Lemons are stronger acids than soft drinks or milk, as indicated by their lower pH value, which is farther from the neutral pH of 7.
- ⚗️ The pH of blood is crucial for life, with a natural pH of about 7.4. Changes to this pH can lead to protein breakdown and potentially fatal consequences if extreme or prolonged.
Q & A
What is a polar molecule?
-A polar molecule is a molecule where one area of the molecule is slightly positive while another area of the same molecule is slightly negative, resulting in an uneven distribution of charge.
How does the polarity of water molecules contribute to their structure?
-The polarity of water molecules is due to oxygen atoms pulling electrons from hydrogen atoms, resulting in a molecule with a slightly negative oxygen end and a slightly positive hydrogen end. This leads to the formation of hydrogen bonds between water molecules.
What is cohesion in the context of water molecules?
-Cohesion is the property where water molecules stick to each other due to hydrogen bonding. It's the force that holds water molecules together, allowing phenomena like a paper clip floating on water.
What is adhesion and how does it relate to water?
-Adhesion is the property where water sticks to other substances. An example of adhesion is water sticking to the walls of a glass, creating a meniscus. It's due to water's polarity, which allows it to form bonds with other materials.
Why does water have a high specific heat?
-Water has a high specific heat because it's a polar molecule with strong hydrogen bonds. It takes a lot of energy to break these bonds and increase the temperature of water, which helps to maintain a constant body temperature in living organisms.
How does the pH scale relate to the properties of water?
-The pH scale measures the concentration of hydrogen ions in a solution. Water, being a very weak base with a pH around 7.4, is crucial for life because it helps maintain the balance of chemical reactions in our bodies.
What is a solution and what are its two main components?
-A solution is a homogeneous mixture where one substance (the solute) dissolves in another (the solvent). The solute can be atoms, ions, or molecules, and the solvent is typically water.
How does the consumption of alcohol affect blood pH?
-Alcohol consumption can lead to an increase in hydrogen ions in the blood, causing the blood pH to become more acidic. Excessive alcohol intake can lead to alcohol poisoning and potentially death due to the disruption of internal chemical reactions.
What is the significance of the meniscus in a graduated cylinder?
-The meniscus is the curve seen at the top of a liquid in a graduated cylinder due to adhesion. When measuring the volume of a liquid, it's important to read the level at the bottom of the meniscus to get an accurate measurement.
How does the process of centrifugation separate the components of blood?
-Centrifugation uses high-speed spinning to separate the components of blood based on their densities. The denser components, like red blood cells, move to the bottom, while lighter components, like plasma, remain at the top.
Why is the pH of blood important for human health?
-The pH of blood is crucial for maintaining proper physiological functions. A pH of around 7.4 indicates a slightly basic environment, which is necessary for the normal functioning of proteins and enzymes in the body. Deviations from this pH can lead to serious health effects or even death.
What happens when substances dissolve in water and release hydrogen ions?
-When substances dissolve in water and release hydrogen ions, the solution becomes acidic. This is because acids have a higher concentration of hydrogen ions, which can disrupt the pH balance of the solution.
Outlines
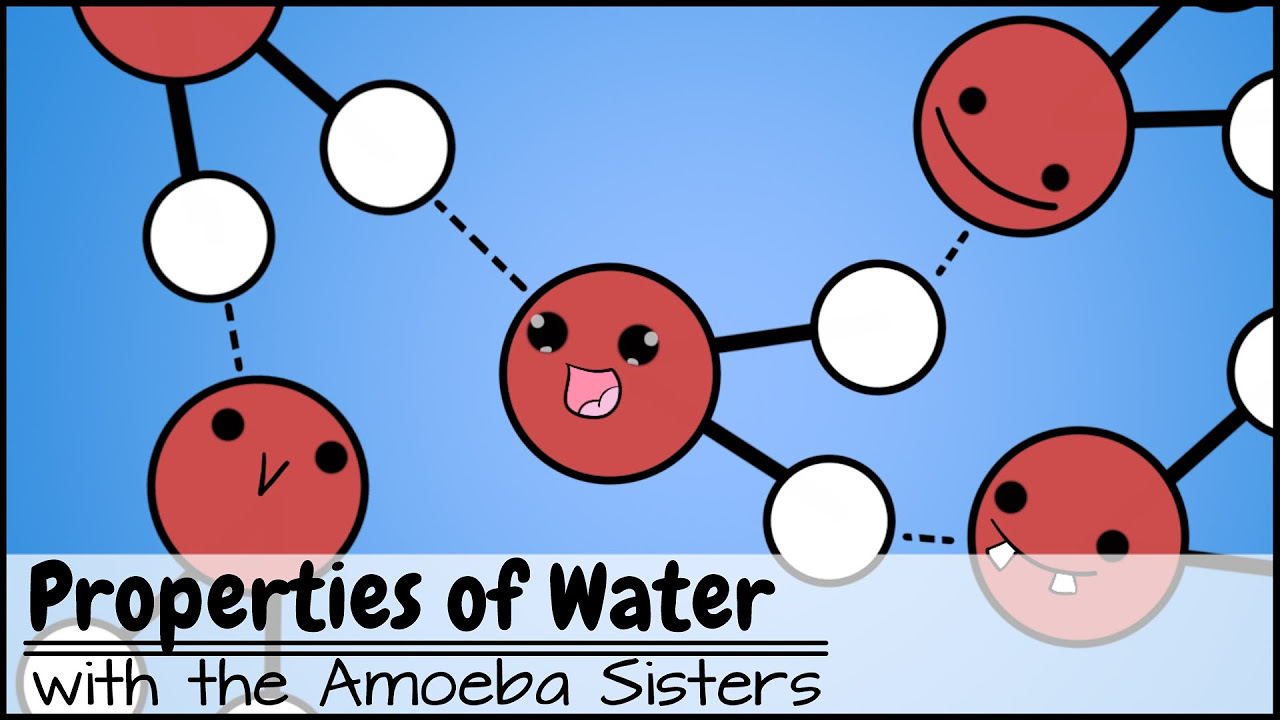
🌊 Understanding Water's Polarity
This paragraph explains the fundamental chemistry of water, emphasizing its polar nature. Water is composed of hydrogen and oxygen, with oxygen having a tendency to attract electrons from hydrogen, resulting in a molecule with a slightly positive side (hydrogen) and a slightly negative side (oxygen). This polarity leads to the formation of hydrogen bonds between water molecules, which is crucial for many of water's unique properties, such as its high specific heat capacity that allows it to resist temperature changes. The paragraph also visually describes how water molecules arrange in layers due to these hydrogen bonds.
🔬 Water's Cohesion, Adhesion, and Solutions
The second paragraph delves into water's properties of cohesion and adhesion. Cohesion is the attraction between water molecules due to hydrogen bonding, which is demonstrated by the ability of a paper clip to float on water's surface. Adhesion is the attraction of water molecules to other substances, exemplified by the meniscus effect observed in a graduated cylinder. The paragraph also introduces the concept of solutions, where a solute dissolves in a solvent, using everyday examples like Kool-Aid and lemonade, and contrasts them with biological solutions like blood.
🧬 Blood as a Solution and the pH Scale
This section discusses blood as an example of a solution, which can be separated into its components through centrifugation, revealing plasma (the solvent), white blood cells, platelets, and red blood cells (the solutes). The pH scale is introduced as a measure of the concentration of hydrogen ions in a solution, with acids having a pH below 7 and bases above 7. Blood's natural pH is around 7.4, making it a weak base, and it is crucial for maintaining life as deviations from this pH can lead to the breakdown of proteins and potentially death. The importance of the pH of blood is highlighted, and the potential dangers of alcohol abuse, which can alter blood pH and cause severe health issues, are also mentioned.
🚫 The Risks of Altering Blood pH
The final paragraph warns about the dangers of changing the pH of blood, particularly through alcohol consumption. It explains how alcohol increases the concentration of hydrogen ions in the blood, leading to a more acidic pH. This shift can disrupt internal chemical reactions and potentially result in alcohol poisoning and death. The paragraph also touches on the role of the kidneys in maintaining the blood's pH by removing excess hydrogen ions. However, individuals with chronic alcohol abuse may suffer from kidney damage, which can impair this regulatory function and lead to serious health consequences.
Mindmap
Keywords
💡Polar Molecule
💡Hydrogen Bond
💡Specific Heat
💡Cohesion
💡Adhesion
💡Solute
💡Solvent
💡pH Scale
💡Acid
💡Base
💡Meniscus
Highlights
Water is a polar molecule with distinct positive and negative charges within the same molecule.
Oxygen atoms in water molecules 'hog' electrons from hydrogen, resulting in a negative charge on oxygen and a positive charge on hydrogen.
The polarity of water leads to the formation of hydrogen bonds between water molecules.
Water's high specific heat capacity allows it to resist temperature changes, which is crucial for maintaining a constant body temperature.
Cohesion is the property that allows water molecules to stick to each other, demonstrated by a paper clip floating on water's surface.
Adhesion is the property that allows water to stick to other substances, as seen in the meniscus effect in a graduated cylinder.
Solutions are mixtures where a solute dissolves in a solvent; water is often the solvent.
The pH scale measures the concentration of hydrogen ions in a solution, with acids below 7 and bases above 7.
Blood is an example of a biological solution, with plasma as the solvent and various molecules as solutes.
Alcohol consumption can alter blood pH, potentially leading to serious health effects due to the accumulation of hydrogen ions.
The kidneys play a role in maintaining blood pH by removing excess hydrogen ions.
Chronic alcohol abuse can lead to kidney damage, impairing the body's ability to regulate blood pH.
The video provides an educational explanation of water's chemical properties and their implications for biological systems.
The hydrogen bonding in water is crucial for its many unique properties, including its surface tension.
The video uses simple demonstrations, like a paper clip on water, to illustrate complex scientific concepts.
Understanding the principles of solutions is fundamental to grasping various biological and chemical processes.
The video emphasizes the importance of pH balance in biological systems, particularly in maintaining healthy blood conditions.
The consequences of disrupting the body's pH balance, such as through alcohol abuse, are explored in the video.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: