Water: A Polar Molecule
TLDRMr. Andersen's video explores the unique properties of water as a polar molecule, emphasizing its crucial role in sustaining life. He explains how the unequal sharing of electrons between oxygen and hydrogen atoms results in partial charges, leading to hydrogen bonding and polarity. This polarity underpins water's remarkable behaviors, such as high specific heat, surface tension, and its ability to dissolve many substances, which are vital for life. The video also touches on water's significance in moderating climate and the search for extraterrestrial life.
Takeaways
- 💧 Water is a polar molecule due to the unequal sharing of electrons between oxygen and hydrogen atoms.
- 🔝 Oxygen is highly electronegative, which means it has a strong tendency to attract electrons towards itself.
- 🧲 The electronegativity of elements increases across the periodic table due to an increase in the number of protons, but decreases down the table due to increased electron shielding.
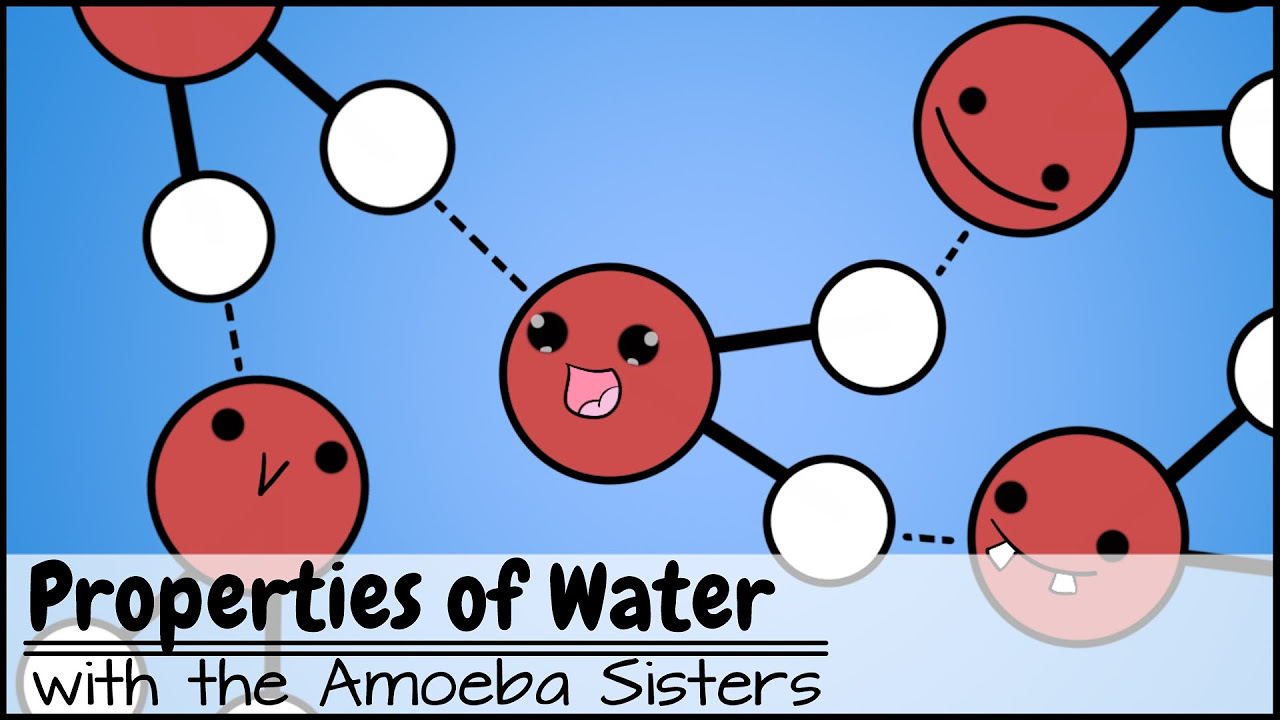
- 🌐 Water molecules exhibit partial positive and negative charges, which creates a dipole moment and allows for hydrogen bonding between molecules.
- 🔗 Hydrogen bonds are a result of the attraction between the positive hydrogen of one water molecule and the negative oxygen of another, forming a dotted line representation.
- 🌊 Cohesion is a property of water where molecules stick together due to hydrogen bonding, which contributes to surface tension.
- 🌿 Capillary action is the ability of water to rise in narrow spaces due to adhesion to the sides and cohesion among water molecules.
- 🔥 Water has a high specific heat capacity, meaning it can absorb a lot of heat without a significant increase in temperature, which moderates climate.
- ❄️ Ice floats on water because the hydrogen bonds form a less dense, three-dimensional lattice structure when water freezes.
- 🧪 Water is an excellent solvent for polar and ionic substances, but not for non-polar substances like oils, due to its polar nature.
- 🌍 The search for extraterrestrial life often focuses on the presence of water, as it is essential for life as we know it, with places like Europa being of particular interest.
Q & A
What does it mean for a molecule to be polar?
-A polar molecule is one where the electrons are shared unequally, resulting in a separation of charge within the molecule, creating a partial positive and a partial negative charge.
Why is oxygen considered highly electronegative?
-Oxygen is highly electronegative because it has a strong tendency to pull electrons towards itself due to its high number of protons in the nucleus and fewer electron shells, which results in a stronger attraction for the shared electrons.
How does the electronegativity change as we move across the periodic table?
-As we move across the periodic table, electronegativity increases because the number of protons in the nucleus increases, enhancing the positive charge and thus attracting electrons more strongly.
Why doesn't electronegativity increase as we move down the periodic table?
-Electronegativity does not increase as we move down the periodic table because the additional electron shells provide more shielding, reducing the effective nuclear charge on the outermost electrons.
What is the significance of the partial positive and partial negative charges in water?
-The partial positive and negative charges in water create a dipole moment, which allows water molecules to form hydrogen bonds with each other, influencing many of water's unique properties.
What is cohesion and how is it related to water's polarity?
-Cohesion is the attraction between molecules of the same substance, in this case, water. It is related to water's polarity because the hydrogen bonds formed due to the partial charges cause water molecules to cling together.
Can you explain capillary action and its connection to water's polarity?
-Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of external forces. It is connected to water's polarity because the hydrogen bonding between water molecules and the surfaces they come into contact with allows water to be pulled up against gravity in small tubes.
How does water's high specific heat capacity affect temperature regulation?
-Water's high specific heat capacity means it can absorb or release a large amount of heat with little change in temperature. This property helps in moderating temperatures in environments, such as the climate around bodies of water like Seattle.
Why does ice float on water, and what role do hydrogen bonds play in this?
-Ice floats on water because as water freezes, the hydrogen bonds form a hexagonal lattice structure that increases the volume and decreases the density of ice, making it less dense than liquid water.
How does water's polarity make it an effective solvent?
-Water's polarity makes it an effective solvent because the partial charges on water molecules can interact with and surround charged particles or polar molecules, allowing them to dissolve in water.
What is the principle of 'like dissolves like' and how does it apply to water?
-'Like dissolves like' is a principle stating that polar substances dissolve well in polar solvents, and nonpolar substances in nonpolar solvents. Water, being polar, dissolves other polar substances easily, such as salts and sugars, but not nonpolar substances like oils.
Outlines
🌊 The Polar Nature of Water
Mr. Andersen introduces the concept of water being a polar molecule, explaining that its polarity arises from the unequal sharing of electrons between oxygen and hydrogen atoms. Oxygen's high electronegativity pulls electrons towards itself, creating partial positive and negative charges within the water molecule. This results in a molecular structure with distinct poles, akin to a magnet, which influences how water molecules interact with each other, forming hydrogen bonds. These bonds are crucial for the cohesion of water, demonstrated by surface tension and the formation of droplets in space, and are fundamental to water's behavior and its role in supporting life on Earth.
🌡 Water's Unique Properties and Implications
This paragraph delves into the various properties of water that stem from its polarity. Cohesion and adhesion are highlighted as key properties, with examples such as the surface tension of water and capillary action, which allows water to rise in narrow tubes, including the xylem in trees. The high specific heat capacity of water is discussed, explaining how it moderates temperature fluctuations, as illustrated by the climate comparison between Bozeman and Seattle. The paragraph also touches on water's ability to dissolve substances, demonstrated through a simulation of salt dissolving in water, due to the interaction between the ions and the polar water molecules. The discussion concludes with the observation that water's polarity makes it an excellent solvent for polar substances, in contrast to non-polar substances like fats, which do not dissolve easily. The significance of water in the search for extraterrestrial life, particularly on Europa, one of Jupiter's moons, is also mentioned.
Mindmap
Keywords
💡Polar Molecule
💡Electronegativity
💡Hydrogen Bond
💡Cohesion
💡Capillary Action
💡Specific Heat
💡Evaporation
💡Solvent
💡Like Dissolves Like
💡Nonpolar
💡Iceberg Calving
Highlights
Water is a polar molecule due to unequal electron sharing, leading to unique behaviors essential for life on Earth.
A water molecule consists of one oxygen and two hydrogen atoms, with oxygen being highly electronegative, pulling electrons towards it.
Electronegativity increases across the periodic table due to an increase in protons, enhancing the pull on electrons.
Descending the periodic table, electron levels shield the pull of protons, which is why electronegativity increases upward.
The electronegativity of oxygen creates partial positive and negative charges within the water molecule, forming a polar nature.
Water molecules can be visualized with imaginary fields around them, similar to a magnet, with poles of positive and negative charges.
The orientation of water molecules is driven by the attraction between the positive hydrogen and negative oxygen of adjacent molecules, forming hydrogen bonds.
Cohesion, the attraction between water molecules, results in high surface tension and is demonstrated by water forming a globule in space.
Capillary action is the upward movement of water in narrow tubes due to adhesion to the tube's surface and cohesion among water molecules.
Water's high specific heat capacity makes it difficult to change its temperature, which is crucial for moderating climate and body temperature.
The property of ice floating is due to the formation of a three-dimensional hydrogen bond matrix that decreases density as water freezes.
Water's polarity makes it an excellent solvent, capable of dissolving many substances, which is vital for biological processes.
The 'like dissolves like' principle explains why polar substances dissolve easily in water, while non-polar substances like oil do not.
Water's ability to dissolve substances is demonstrated through a simulation showing sodium chloride breaking into ions in water.
The search for extraterrestrial life often focuses on the presence of water, as it is considered a key ingredient for life.
Europa, a moon of Jupiter, is considered a potential site for life due to the possibility of a subsurface liquid water ocean.
The polar nature of water is fundamental for the existence of life on Earth and is a critical factor in the search for life beyond our planet.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: