(a) Protons, neutrons and electrons in terms of their relative charges and masses
TLDRThis video from Understand Academy explores Learning Outcome II on atomic structure, focusing on identifying and describing protons, neutrons, and electrons in terms of their relative charges and masses. It defines the atom as the fundamental building block of all matter and explains that atoms can be divided into subatomic particles. The video clarifies that the electron is the smallest indivisible subatomic particle and provides the relative charges (+1 for protons, 0 for neutrons, -1 for electrons) and masses, emphasizing the electron's negligible mass compared to protons and neutrons. The summary encourages students to understand these concepts for the GCH Chemistry syllabus.
Takeaways
- 🌐 The atom is the smallest particle of an element that maintains its chemical properties and is the building block of all matter in the universe.
- 🔬 Atoms can be divided into three types of subatomic particles: protons, neutrons, and electrons.
- 🚀 Protons can be further divided into smaller particles known as quarks, specifically two up quarks and one down quark.
- 🤹 Neutrons also consist of quarks, with two down quarks and one up quark.
- 🌀 Electrons, as currently understood, are the smallest subatomic particles and cannot be subdivided further.
- ⚡ The symbol for a proton is 'p+', for a neutron 'n', and for an electron 'e-', representing their respective charges.
- 🔋 The electrical charge of a proton is +1.6 × 10^-19 coulombs, a neutron has no charge, and an electron has a charge of -1.6 × 10^-19 coulombs.
- ⚖️ The relative electrical charges of protons, neutrons, and electrons are +1, 0, and -1, respectively.
- 📊 The mass of a proton and neutron are very close, while the mass of an electron is significantly smaller, to the point of being negligible in comparison.
- 📐 The relative masses of protons, neutrons, and electrons are in the ratio of 1:1:1/1840, highlighting the electron's minimal mass.
- 📚 The learning outcome requires knowledge of the relative charges and masses of subatomic particles, not their actual values in grams or coulombs.
Q & A
What is the definition of an atom according to the video?
-An atom is defined as the smallest particle of an element that maintains the chemical properties of that element. It is considered the building block of all material that exists in the universe.
Can atoms be divided into smaller particles?
-Yes, atoms can be divided into smaller particles known as subatomic particles, which include protons, neutrons, and electrons.
What are the three types of subatomic particles?
-The three types of subatomic particles are protons, neutrons, and electrons.
What are the sub-particles that make up a proton?
-A proton is made up of smaller particles called quarks, specifically two up quarks and one down quark.
How is a neutron composed in terms of quarks?
-A neutron is composed of three quarks: two down quarks and one up quark.
Is the electron considered a fundamental particle?
-As we know today, the electron is the smallest subatomic particle that cannot be further divided into smaller particles.
What are the symbols used to represent protons, neutrons, and electrons?
-The symbols are 'p+' for protons, 'n' for neutrons, and 'e-' for electrons.
What are the relative electrical charges of protons, neutrons, and electrons?
-The relative electrical charges are +1 for protons, 0 for neutrons, and -1 for electrons.
How are the masses of protons, neutrons, and electrons typically compared?
-The relative masses are compared in a ratio where the mass of the proton and neutron are considered as one unit each, and the mass of the electron is negligible, often stated as 1/1840 of the proton's mass.
What is the significance of the electron's mass being negligible compared to protons and neutrons?
-The electron's mass being negligible compared to protons and neutrons means that in most calculations and considerations, the mass of an atom is primarily determined by the number of protons and neutrons, not the electrons.
What does the video suggest for students to do after learning about the relative charges and masses of subatomic particles?
-The video suggests that students should be able to identify and describe protons, neutrons, and electrons in terms of their relative charges and masses, which is part of the learning outcome II from the topic of atomic structure.
Outlines
🌌 Introduction to Atomic Structure
This paragraph introduces the topic of atomic structure from the JCH new chemistries universe, focusing on learning outcome II. The video aims to help candidates identify and describe protons, neutrons, and electrons in terms of their relative charges and masses. It begins by defining an atom as the smallest particle of an element that maintains its chemical properties and emphasizes the atom's role as the fundamental building block of all matter in the universe. The paragraph also touches on the possibility of atoms being divided into smaller particles, known as subatomic particles: protons, neutrons, and electrons. It mentions that protons and neutrons can be further divided into quarks, but electrons are currently considered indivisible. The paragraph sets the stage for a deeper dive into the properties of these subatomic particles.
Mindmap
Keywords
💡Atom
💡Subatomic Particles
💡Proton
💡Neutron
💡Electron
💡Electric Charge
💡Relative Charge
💡Mass
💡Relative Mass
💡Quarks
💡Learning Outcome
Highlights
Introduction to the topic of atomic structure from JCH new chemistries universe.
Learning outcome II: Identifying and describing protons, neutrons, and electrons in terms of relative charges and relative masses.
Definition of an atom as the smallest particle of an element maintaining its chemical properties.
Atoms are the building blocks of all material in the universe.
Atoms can be divided into three types of subatomic particles: protons, neutrons, and electrons.
Protons can be divided into smaller particles called quarks, specifically two up quarks and one down quark.
Neutrons are also composed of three quarks: two down quarks and one up quark.
Electrons are currently considered the smallest subatomic particle and cannot be divided further.
Symbols for subatomic particles: p+ for protons, n for neutrons, and e- for electrons.
Electrical charge of subatomic particles measured in coulombs: proton (+1.6 x 10^-19), neutron (0), and electron (-1.6 x 10^-19).
Relative electrical charges: proton (+1), neutron (0), and electron (-1).
Masses of subatomic particles: proton and neutron have similar masses, while the electron's mass is much smaller and often considered negligible.
Relative masses of subatomic particles: proton (1 unit), neutron (1 unit), and electron (1/1840 unit).
Summary of the learning outcome: identifying and describing protons, neutrons, and electrons by their relative charges and masses.
Encouragement to like and subscribe for more videos on JCH chemistry topics.
Transcripts
Browse More Related Video

TEAS 7 Chemistry: Introduction to Atoms

Basic Atomic Structure: A Look Inside the Atom

How to find the number of protons, neutrons, and electrons from the periodic table

Inside Atoms: The Proton Numbers
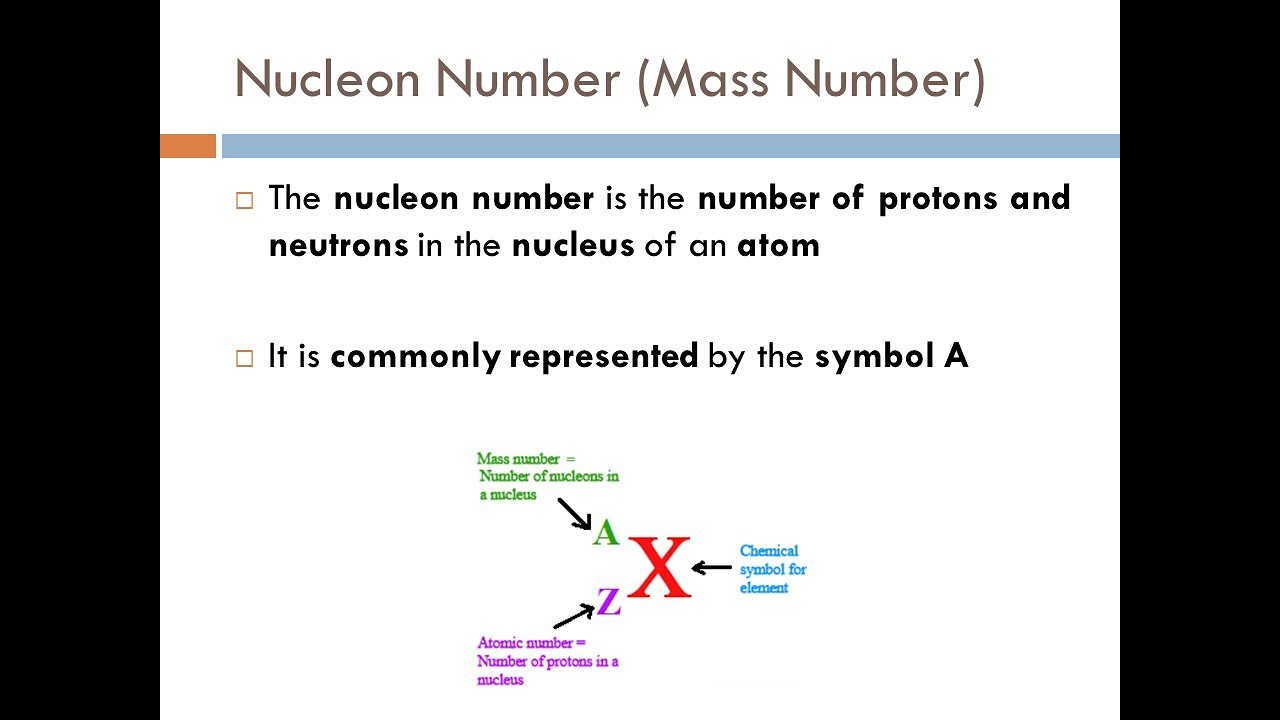
Learning Outcomes (d) and (e) from Atomic Structure - JC H2 Chemistry

(b) Deduce the behaviour of beams of protons, neutrons and electrons in an electric field
5.0 / 5 (0 votes)
Thanks for rating: