[H2 Chemistry] 2021 Topic 3 The Gaseous State
TLDRThe lecture series delves into the third topic of JC1 studies, focusing on the behavior of gases and the concept of ideal gas states. It revisits fundamental principles like Boyle's and Charles' laws, introduces Avogadro's law, and explains the ideal gas equation (PV=nRT). The course also covers the real-world applications of these principles, including calculations involving gas volumes, pressures, and temperatures. It explores the deviations of real gases from ideal behavior under various conditions and introduces the Maxwell-Boltzmann distribution of molecular speeds. Practical aspects like gas collection techniques and the use of graduated gas syringes are also discussed.
Takeaways
- 📚 Topic three on gaseous states covers relationships between pressure, volume, and temperature, as known from Boyle's and Charles' laws.
- 📝 The ideal gas law (PV=nRT) combines these relationships and is essential for understanding gas behavior.
- 🔍 Common student mistakes include unit conversion errors and confusion in combining different gas laws.
- ⚗️ Understanding the historical development of gas laws by chemists like Boyle, Charles, and Gay-Lussac is crucial.
- 🧪 Avogadro's law states equal volumes of gases at the same temperature and pressure contain the same number of particles.
- 📏 Units of pressure include pascal (Pa) and atmosphere (atm), with conversions necessary for calculations.
- 📈 Simple gas laws and the ideal gas law help determine gas properties like pressure, volume, temperature, and moles in different conditions.
- 🔬 Dalton's law of partial pressures explains the total pressure as the sum of individual gas pressures in a mixture.
- 🔄 Real gases deviate from ideal behavior at high pressures and low temperatures due to intermolecular forces.
- 📊 The Maxwell-Boltzmann distribution describes the speed distribution of gas particles, showing broader distributions at higher temperatures.
Q & A
What are the main topics covered in the lecture series on 'Geisha States'?
-The lecture series covers learning objectives from 3a to 3d, including familiar concepts from secondary school physics such as the relationship between pressure and volume, and volume and temperature, also known as Boyle's Law and Charles' Law. It also discusses the Ideal Gas Law (PV=nRT), Avogadro's Law, and the concept of relative molecular mass.
Why might students find it difficult to combine Boyle's Law and Charles' Law?
-Students often find it challenging to combine these laws because they need to use the Ideal Gas Law (PV=nRT) correctly and avoid common mistakes in calculations, especially when it comes to remembering units and deriving the equation from fundamental principles.
What is the significance of the Ideal Gas Law in H2 Chemistry?
-The Ideal Gas Law is crucial in H2 Chemistry as it is important across all areas of the subject, particularly in calculations. It can even be applied in organic chemistry to assume ideal behavior and calculate values such as molar mass from given information.
Who are some of the chemists associated with the development of gas laws?
-Some of the chemists include Robert Boyle, who developed Boyle's Law, and Jacob Charles, who contributed to the understanding of the relationship between volume and temperature, which is part of Charles' Law. Additionally, Amedeo Avogadro is known for Avogadro's Law, which relates to the number of particles in a gas.
What is Avogadro's constant and why is it important?
-Avogadro's constant (represented by 'L') is the number of particles contained in one mole of a substance, exactly defined as 6.02 × 10^23. It is crucial for understanding and deriving future relationships in chemistry, such as those involving the number of moles and the volume of gases.
What are the units for Avogadro's constant and how should they be written?
-The unit for Avogadro's constant is 'mol'. It should not be written as 'mols', even when referring to plural. The constant is represented by the Greek letter 'eta' (H) in data booklets and should be used to represent the amount or number of moles.
What is the definition of relative molecular mass and how is it related to carbon-12?
-Relative molecular mass is defined as the average mass of one molecule of a substance divided by 1/12 the mass of one atom of carbon-12. Carbon-12 is used as the reference standard, and its mass is accurately measured to be 12 atomic mass units.
What are the units used to measure pressure, and how are they related to each other?
-Pressure is traditionally measured in units such as millimeters of mercury and atmospheres. However, the SI unit for pressure is the pascal, defined as one newton per square meter. One atmosphere is exactly 101,325 pascals, which is also equivalent to 760 millimeters of mercury.
What are the two standard conditions for gas measurements mentioned in the script, and what are their respective molar volumes?
-The two standard conditions are Standard Temperature and Pressure (STP) and Room Temperature and Pressure (RTP). At STP, the molar volume is defined as 22.7 dm³ per mole, while at RTP, it is 24 dm³ per mole.
How is the Ideal Gas Law derived from the condition of room temperature and pressure?
-The Ideal Gas Law (PV=nRT) can be derived using the condition of room temperature and pressure, where the pressure is 101,325 pascals, the volume is 24 dm³ (converted to cubic meters), the number of moles (n) is 1.0, and the thermodynamic temperature (T) is 293 kelvins. The gas constant (R) is calculated to be approximately 8.31 joules per mole per kelvin.
Outlines
🔬 Introduction to Gas Laws
The lecturer introduces the topic of gas laws for JC1 students, covering the learning objectives from the syllabus, and highlighting familiar concepts such as Boyle's Law and Charles' Law. Emphasis is placed on understanding the relationships between pressure, volume, and temperature, and the importance of accurate calculations and unit conversions in understanding gas behavior in chemistry.
📐 Avogadro's Law and Molar Concepts
Avogadro's Law is introduced, stating that equal volumes of all gases at the same temperature and pressure contain the same number of particles. The concept of the mole is defined as 6.02 x 10^23 particles, and the importance of using the Avogadro constant correctly is emphasized. Units of measurement, such as moles, and the significance of maintaining consistency in calculations are discussed.
📏 Pressure and Volume Relationships
This section covers the definition and measurement of pressure, including traditional and SI units. The importance of converting units and understanding the relationships between pressure, volume, and temperature is highlighted. Boyle's Law and Charles' Law are revisited, emphasizing the mathematical relationships and graphical representations of these gas laws.
📊 Graphical Analysis of Gas Laws
The discussion moves to the graphical representation of gas laws, focusing on Boyle's and Charles' laws. The lecturer explains how to plot graphs of pressure vs. volume and volume vs. temperature, illustrating the inverse and direct proportionality of these relationships. The significance of thermodynamic temperature and the conversion between Celsius and Kelvin is emphasized.
🔧 Ideal Gas Equation
The ideal gas equation (PV = nRT) is introduced as a critical relationship in understanding gas behavior. The importance of remembering this equation, despite it not being in the data booklet, is stressed. The lecturer explains how to use the ideal gas equation in various calculations, such as determining pressure, volume, temperature, and amount of gas.
📉 Derivation and Application of Ideal Gas Equation
The lecturer demonstrates the derivation of the ideal gas constant (R) using standard temperature and pressure conditions. The practical application of the ideal gas equation is shown through examples, such as calculating the volume of gas at given conditions. The significance of using appropriate units in these calculations is reiterated.
🧪 Exercises on Gas Laws
Several exercises are presented to reinforce the understanding of gas laws. The lecturer guides students through calculations involving gas laws, emphasizing the importance of unit conversion and careful application of the ideal gas equation. Examples include determining the molar mass of gases and understanding the behavior of gas mixtures.
📐 Combined Gas Law
The combined gas law, which relates pressure, volume, and temperature when the number of moles is constant, is introduced. The lecturer explains how to derive this relationship and apply it to solve problems involving changing conditions. The importance of understanding the conservation of mass in these calculations is highlighted.
🌡️ Real Gas Behavior and Deviations
The behavior of real gases is discussed, focusing on how they deviate from ideal gas behavior at high pressures and low temperatures. The lecturer explains the significance of intermolecular forces and the conditions under which real gases approximate ideal behavior. Graphical representations of these deviations are also presented.
📊 Analyzing Real Gas Behavior
Exercises are provided to help students analyze real gas behavior using the combined gas law and ideal gas equation. The importance of understanding the assumptions behind these equations and the conditions that lead to deviations is emphasized. Examples include calculating the volume of gas bubbles and understanding the compression of gas mixtures.
🧩 Graphical Representation of Gas Laws
The lecturer discusses the importance of graphical representation in understanding gas laws. The relationship between pressure, volume, and temperature is further explored through various examples. The importance of accurately interpreting and plotting these relationships is emphasized, and students are encouraged to practice these skills.
📈 Dalton's Law of Partial Pressure
Dalton's Law of Partial Pressure is introduced, explaining how the total pressure of a gas mixture is the sum of the partial pressures of each component gas. The lecturer discusses the concept of mole fraction and how it is used to calculate partial pressures in gas mixtures. Examples and exercises are provided to reinforce understanding.
📐 Calculating Partial Pressures
The lecturer provides detailed examples of calculating partial pressures using Dalton's Law. Exercises include determining the partial pressures of oxygen and helium in a gas mixture. The importance of accurate unit conversion and understanding the mole fraction in these calculations is emphasized.
📊 Interpreting Real Gas Behavior
Further exercises on interpreting real gas behavior are presented, focusing on the deviations from ideal gas behavior. The lecturer explains how to use plots of PV against P to identify gases based on their intermolecular forces. The significance of understanding these deviations in practical applications is highlighted.
📈 Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution curve is introduced to explain the statistical distribution of molecular speeds in a gas. The lecturer discusses how temperature affects the distribution of kinetic energy among gas particles. The implications of this distribution for understanding gas behavior and chemical kinetics are also explored.
📊 Statistical Distribution of Molecular Speeds
Further details on the Maxwell-Boltzmann distribution are provided, emphasizing how the distribution curve broadens with increasing temperature. The lecturer explains the significance of this distribution for understanding the kinetic energy of gas particles and its impact on reaction rates in chemical kinetics.
🔬 Collecting and Measuring Gases
The process of collecting and measuring gases in laboratory experiments is discussed. The lecturer explains the importance of using graduated gas syringes and proper setup for gas collection. Practical tips for accurate measurement and common pitfalls to avoid are provided.
🧪 Practical Applications of Gas Collection
Further practical applications of gas collection are explored, including the setup for different types of gas collection methods. The importance of accounting for displaced air and ensuring proper connections in experimental setups is emphasized. Students are encouraged to practice these techniques in laboratory settings.
🌡️ Gas Collection Procedures
Detailed procedures for gas collection in laboratory experiments are provided. The lecturer emphasizes the importance of initial and final readings in gas collection, as well as the use of proper apparatus. Students are guided through typical setups and reminded of key points to ensure accurate results.
Mindmap
Keywords
💡Gas Laws
💡Ideal Gas
💡Avogadro's Law
💡Molar Volume
💡Pressure
💡Thermodynamic Temperature
💡Mole Fraction
💡Dalton's Law of Partial Pressures
💡Kinetic Particle Theory
💡Real Gases
💡Gas Collection
Highlights
Introduction to the third topic in JC1 studies focusing on the states of gases.
Review of learning objectives from syllabus documents 3A to 3D, including familiar concepts from secondary school physics.
Explanation of the relationship between pressure and volume, known as Boyle's Law, and volume and temperature, known as Charles' Law.
Discussion on the difficulty students face when combining Boyle's and Charles' Laws using the ideal gas equation PV=nRT.
Importance of understanding the ideal gas equation for calculations across various chemistry areas, including organic chemistry.
Introduction to the concept of Avogadro's Law and its significance in deriving future relationships.
Clarification on the use of the Avogadro constant (L) and its correct representation in calculations.
Explanation of relative molecular mass and its definition based on the average mass of a molecule or atom.
Introduction to units of pressure, including the traditional unit of mercury barometer and the SI unit Pascal.
Conversion between different units of pressure, such as atmospheres, pascals, and bars.
Discussion on standard temperature and pressure (STP) and room temperature and pressure (RTP) conditions for gas samples.
Graphical representation of Boyle's Law and Charles' Law, including inverse proportionality and direct proportionality relationships.
Application of the ideal gas equation in exercises to determine variables like volume and molar mass.
Derivation and explanation of the ideal gas constant (R) and its units in relation to pressure, volume, and temperature.
Use of the ideal gas equation in combined gas calculations and the importance of maintaining consistent units.
Introduction to Dalton's Law of Partial Pressures and its application in calculating total and partial pressures in a mixture of gases.
Discussion on the assumptions of ideal gas behavior, including negligible size and intermolecular forces of gas particles.
Conditions for real gases to behave ideally, such as low pressure and high temperature, and their implications.
Explanation of real gas deviations from ideal behavior at high pressures and low temperatures.
Illustration of real gas behavior compared to ideal gas through graphical representation and analysis.
Overview of gas collection techniques, including upward and downward displacement methods, and their laboratory applications.
Transcripts
Browse More Related Video

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
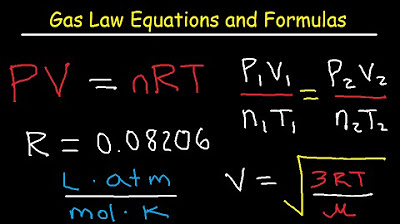
Gas Laws - Equations and Formulas
Ideal Gas Problems: Crash Course Chemistry #13
Kinetic Molecular Theory of Gases - Practice Problems

The Ideal Gas Law: Crash Course Chemistry #12
9.2 Gas Laws including the Ideal Gas Law | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: