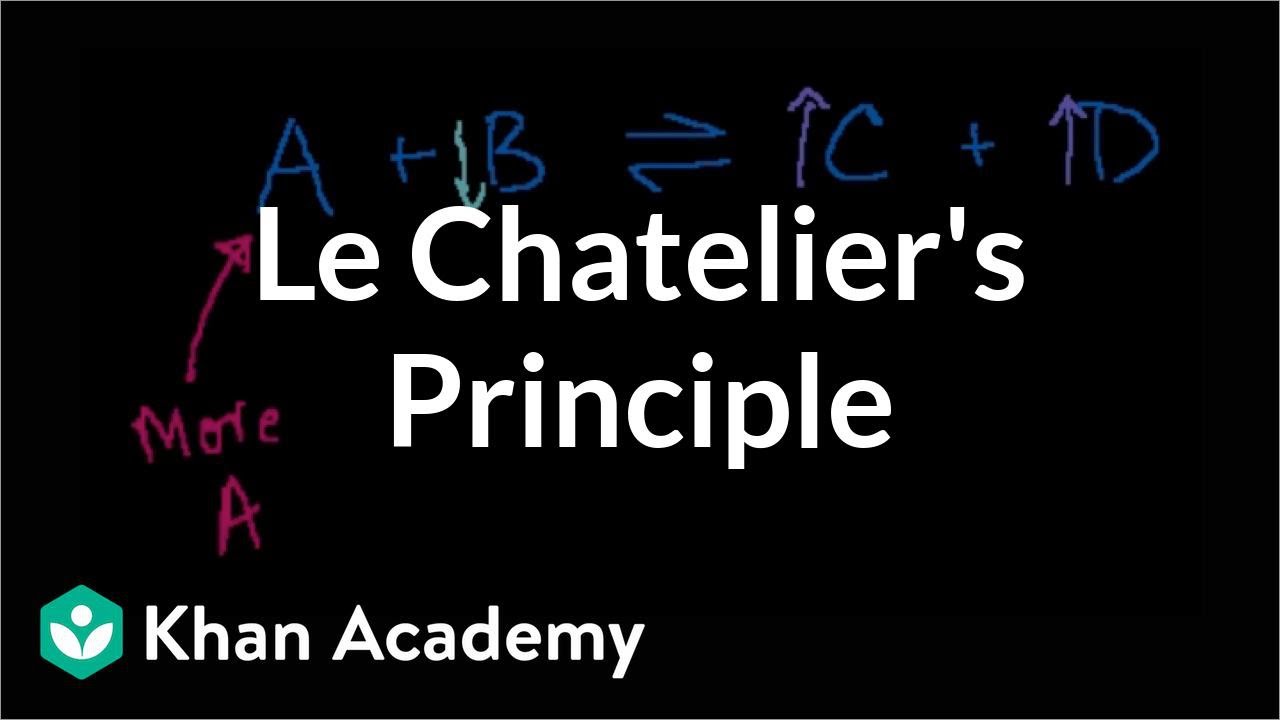
Le Chatelier's Principle
TLDRProfessor Dave's video script delves into Le Chatelier's principle, explaining how a system at equilibrium responds to various forms of stress. The principle states that when a system is disturbed, it will adjust to counteract the change and restore balance. The video covers three primary types of stress: altering the concentration of reactants or products, which speeds up the forward or reverse reaction to reestablish equilibrium; changing the temperature, which affects exothermic and endothermic reactions differently, as heat can be considered a product or reactant; and modifying the volume or pressure, which impacts gaseous systems and causes a shift towards the side with fewer particles to reduce pressure. The comprehensive explanation is designed to enhance understanding of chemical equilibrium dynamics and encourages viewers to subscribe for more educational content.
Takeaways
- 🔍 Le Chatelier's principle states that a system at equilibrium will adjust to counteract any changes or stresses applied to it.
- 🧪 Adding or removing reactants/products can cause the equilibrium to shift, with the forward reaction speeding up to use up excess reactants or the reverse reaction to produce more of the removed species.
- 🌡️ Changing the temperature can affect equilibrium, with exothermic reactions shifting in the direction that absorbs heat (towards the side that releases heat) when the system is heated, and endothermic reactions shifting in the direction that releases heat (towards the side that absorbs heat).
- ⚗️ The sign of delta H (change in enthalpy) determines if a reaction is exothermic (negative) or endothermic (positive), which influences how temperature changes affect the equilibrium.
- 🌡️ Increasing temperature generally causes the equilibrium to shift towards the side that absorbs heat, while cooling shifts it towards the side that releases heat.
- 🎈 Changes in volume or pressure, especially in systems involving gases, can also shift the equilibrium. According to Boyle's law, a decrease in volume increases pressure.
- ➡️ If there's a difference in the number of moles of gas on either side of the equilibrium, a change in volume or pressure will cause the system to shift towards the side with fewer moles to reduce pressure.
- 🔄 Decreasing the volume (or increasing pressure) favors the side with fewer gas particles, while increasing the volume (or decreasing pressure) favors the side with more gas particles.
- 📉 In the given example, a diatomic molecule and two monoatomic species are at equilibrium; a shift to the left (towards the diatomic molecule) would decrease the number of particles and thus the pressure.
- 📈 Conversely, increasing the volume would cause the equilibrium to shift to the right (towards the monoatomic species) to increase the number of particles and regain pressure.
- 📚 Understanding these principles is crucial for predicting how a system at equilibrium will respond to changes in concentration, temperature, and pressure.
- 📧 The video encourages viewers to subscribe for more tutorials and to reach out with questions via email.
Q & A
What does Le Chatelier's principle state?
-Le Chatelier's principle states that if a stress is induced on a system at equilibrium, the equilibrium will shift to relieve that stress.
How does adding a reactant to a system at equilibrium affect the equilibrium?
-Adding a reactant to a system at equilibrium will unbalance it, causing the forward reaction to speed up and use up the additional reactants, turning them into products to restore equilibrium. The equilibrium is said to have shifted to the right.
What happens if more products are added to a system at equilibrium?
-If more products are added to a system at equilibrium, the equilibrium will shift to the left, favoring the reverse reaction to produce more reactants and restore balance.
How does removing one of the components from a system at equilibrium affect the equilibrium?
-Removing one of the components from a system at equilibrium will cause the equilibrium to shift to produce more of that species to restore balance.
What is the effect of changing the temperature on a chemical equilibrium?
-Changing the temperature affects a chemical equilibrium by shifting it in the direction that absorbs or releases heat, depending on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
How is the heat energy treated in relation to an exothermic or endothermic reaction?
-In an exothermic reaction, heat energy is treated as a product because the reaction releases energy. In an endothermic reaction, heat energy is treated as a reactant because energy must be absorbed for the reaction to proceed.
What is the effect of increasing the temperature on an exothermic reaction?
-Increasing the temperature on an exothermic reaction will cause the equilibrium to shift in the reverse direction to use up the excess heat and relieve the stress.
What is the effect of cooling down a system at equilibrium?
-Cooling down a system at equilibrium will have the opposite effect of increasing temperature. It will shift the equilibrium towards the side that produces more heat, favoring the exothermic direction.
How does changing the volume or pressure affect a gaseous equilibrium?
-Changing the volume or pressure affects a gaseous equilibrium by shifting it towards the side with fewer particles to alleviate the additional pressure when the volume is decreased, or towards the side with more particles to regain lost pressure when the volume is increased.
What is Boyle's law and how does it relate to the pressure in a gaseous system?
-Boyle's law states that the pressure of a gas is inversely proportional to its volume when the temperature is held constant. If the volume decreases, the pressure increases because the gas particles hit the sides of the container more frequently.
Can you provide an example of how the equilibrium shifts when the volume of a gaseous system is decreased?
-If the equilibrium involves a diatomic molecule and two monoatomic species, and the right side has twice as many particles, decreasing the volume will increase the pressure. The equilibrium will shift to the left, favoring the side with fewer particles (the diatomic molecule) to lower the pressure on the container.
What happens if the volume of a gaseous system at equilibrium is increased?
-If the volume of a gaseous system at equilibrium is increased, the pressure decreases. The equilibrium will shift towards the side with more particles to regain some of the lost pressure.
Outlines
🔬 Introduction to Le Chatelier's Principle
Professor Dave introduces Le Chatelier's principle, which states that a system at equilibrium will adjust to counteract any changes or stresses placed upon it. He explains that these stresses can come in the form of altering concentrations of reactants or products, changing the temperature (which affects whether an exothermic or endothermic reaction is favored), or modifying the volume or pressure (as per Boyle's law). The principle is demonstrated through examples, showing how the equilibrium shifts in response to these changes to restore balance.
Mindmap
Keywords
💡Le Chatelier's Principle
💡Equilibrium
💡Stress
💡Concentration
💡Exothermic Reaction
💡Endothermic Reaction
💡Enthalpy (delta H)
💡Volume and Pressure
💡Boyle's Law
💡Moles of Particles
💡Thermochemical Data
Highlights
Le Châtelier's principle states that a system at equilibrium will shift to relieve stress induced by changes.
Adding reactants to a system at equilibrium will cause the forward reaction to speed up, consuming the added reactants and restoring balance.
Increasing product concentration will shift the equilibrium to the left, producing more of the species removed.
Removing a component from the system will cause the equilibrium to shift to produce more of that species to restore balance.
Changing the temperature affects equilibrium based on whether the reaction is exothermic or endothermic.
Exothermic reactions, with a negative delta H, release energy and treat heat as a product.
Endothermic reactions, with a positive delta H, absorb energy and treat heat as a reactant.
Higher temperatures cause a shift in equilibrium to use up excess heat, while cooling shifts it in the opposite direction.
Changing the volume or pressure of a system with gases will also affect the equilibrium position.
Decreasing the volume of a gaseous system increases pressure, causing the equilibrium to shift towards the side with fewer particles.
Increasing the volume decreases pressure, and the equilibrium shifts towards the side with more particles to regain pressure.
Boyle's law is referenced to explain the relationship between volume and pressure in a gaseous system at equilibrium.
The equilibrium shift towards fewer particles can be visualized as atoms fusing together, reducing the number of gas particles and pressure.
The video provides a comprehensive understanding of how different stresses impact the equilibrium of chemical reactions.
The tutorial is aimed at viewers interested in learning about chemical equilibrium and Le Châtelier's principle.
Professor Dave's channel offers more tutorials for those interested in further learning.
Viewers are encouraged to subscribe to the channel and contact Professor Dave with any questions via email.
Transcripts
Browse More Related Video
Le Chatelier's principle | Chemical equilibrium | Chemistry | Khan Academy

Le Chatelier's Principle

Chemistry | Chemical equilibrium | Le Chartelie's Principle

Chapter 6: Equilibrium and Temperature | CHM 214 | 053

ATI TEAS 7 I Complete Chemistry Review Part 2 I
Chemical Equilibria and Reaction Quotients
5.0 / 5 (0 votes)
Thanks for rating: