Le Chatelier's Principle
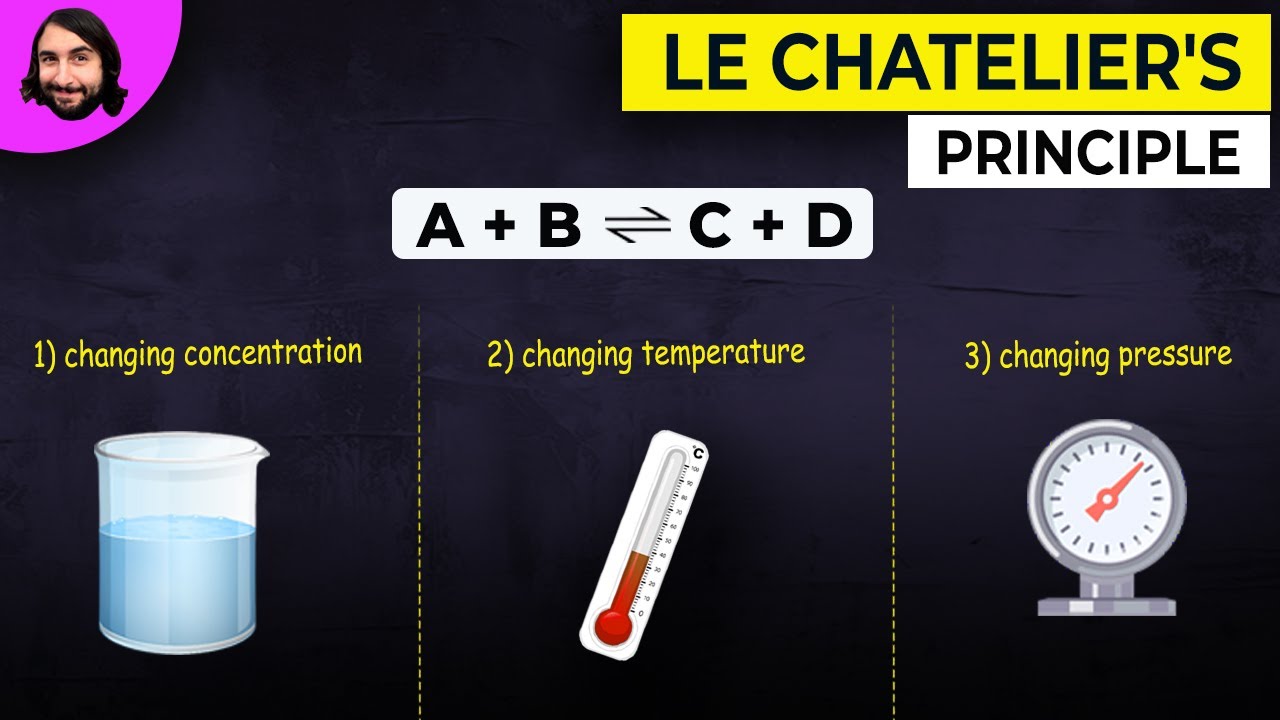
TLDRThe video script discusses Le Chatelier's Principle, which states that when a change is imposed on a system at equilibrium, the system will adjust to counteract the change. It explains this principle with examples of both physical and chemical equilibrium, emphasizing the dynamic nature of chemical equilibrium where the rates of forward and reverse reactions are equal. The video also explores how altering the concentration of reactants or products, as well as conditions like volume and pressure, influences the direction of equilibrium shift. The key takeaway is that the system will always strive to maintain balance by undoing the imposed changes.
Takeaways
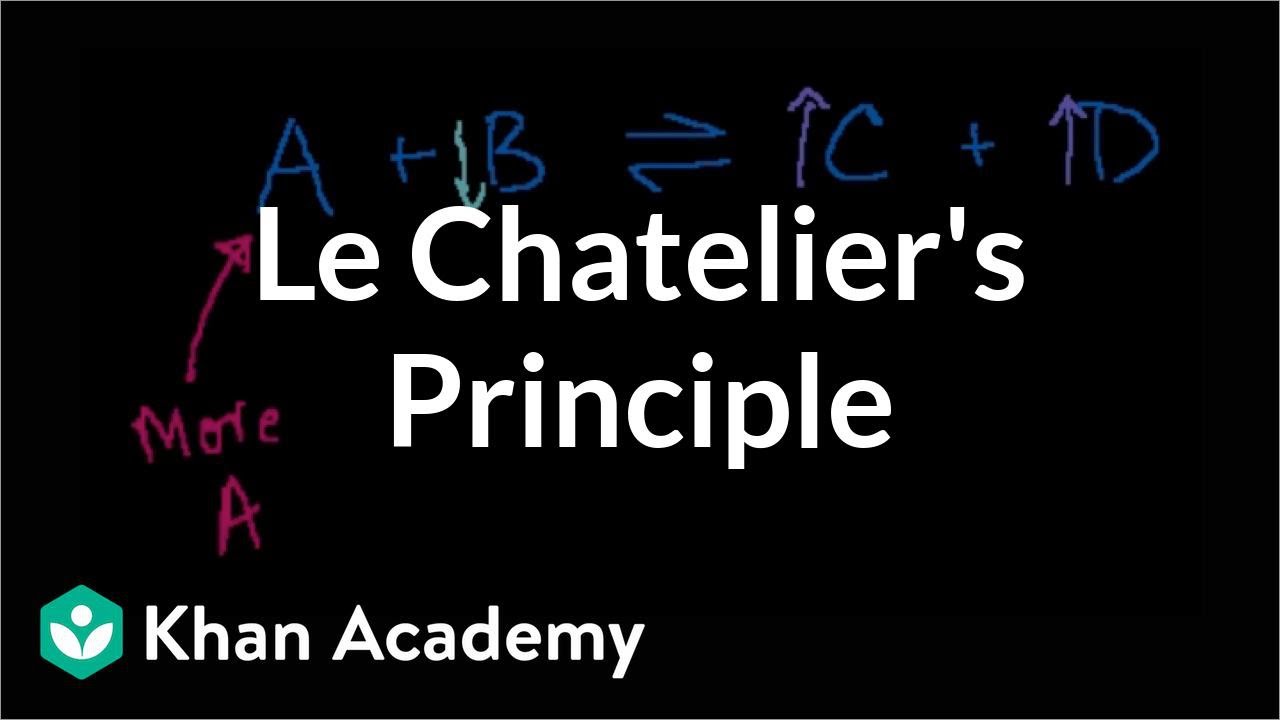
- 📈 Le Chatelier's Principle states that if a change is imposed on a system at equilibrium, the system will adjust to counteract that change.
- 🔄 At equilibrium, a chemical reaction is in a dynamic state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in constant concentrations of products and reactants.
- 🔄 Increasing the concentration of a gaseous reactant (e.g., B) will cause the reaction to shift towards the products (right) to decrease that reactant.
- 🔄 Decreasing the concentration of a gaseous reactant (e.g., B) will cause the reaction to shift towards the reactants (left) to increase that reactant.
- 🔄 Increasing the concentration of a product (e.g., C) will cause the reaction to shift towards the reactants (left) to decrease that product.
- 🔄 Changes in the concentration of solid or liquid reactants or products do not affect the position of equilibrium, as they are not included in the equilibrium constant (K) expression.
- 🌐 Adding a catalyst speeds up the rate of both the forward and reverse reactions but does not change the position of equilibrium, as it does not affect the relative concentrations of products and reactants.
- 📉 Removing a reactant gas (e.g., H2) from the reaction vessel will cause the reaction to shift towards the reactants (left) to produce more of that reactant.
- 🔄 Increasing the volume of the container (decreasing pressure) will cause the reaction to shift towards the side with more moles of gas to increase the total pressure.
- 🔄 Decreasing the volume of the container (increasing pressure) will cause the reaction to shift towards the side with fewer moles of gas to decrease the total pressure.
- 🔗 The net effect of changes in partial pressures is determined by the ratio of gas molecules on each side of the reaction; the side with more moles of gas will dominate the shift in equilibrium.
Q & A
What is Le Chatelier's Principle?
-Le Chatelier's Principle states that when a change is imposed on a system at equilibrium, the system will adjust itself to counteract or reduce the effect of that change.
What happens when a system is at equilibrium?
-When a system is at equilibrium, it is in a state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in constant concentrations of products and reactants.
What is the difference between static and dynamic equilibrium?
-Static equilibrium refers to a state where no movement or change occurs, while dynamic equilibrium, as seen in chemical reactions, involves ongoing forward and reverse reactions at equal rates, maintaining a balance of products and reactants.
How does the system respond when the concentration of reactant B is increased?
-According to Le Chatelier's Principle, increasing the concentration of reactant B will cause the system to try to decrease it by shifting the reaction towards the products (to the right), where the concentration of B will decrease.
What happens if the concentration of product C is increased?
-If the concentration of product C is increased, the system will try to decrease it by shifting the reaction towards the reactants (to the left), where the concentration of C will decrease.
How does the system respond to the removal of reactant B from the reaction vessel?
-Removing reactant B from the reaction vessel will cause the system to try to increase the concentration of B by shifting the reaction towards the reactants (to the left), producing more B.
What is the effect of adding more substance A, which is a solid, to the reaction vessel on the position of equilibrium?
-Adding more substance A, which is a solid, will not affect the position of equilibrium because the equilibrium constant does not depend on the concentration of solids or liquids.
What happens when the volume of the container is increased?
-When the volume of the container is increased, the total pressure decreases. According to Le Chatelier's Principle, the system will try to increase the total pressure by shifting the reaction towards the side with more moles of gas, which is to the left if there are more gas molecules on the left side.
How does the system respond to the decrease in volume of the container?
-When the volume of the container decreases, the total pressure increases. The system will try to decrease the total pressure by shifting the reaction towards the side with fewer moles of gas, which is to the right if there are fewer gas molecules on the right side.
What is the effect of adding a catalyst on the position of equilibrium?
-Adding a catalyst speeds up both the forward and reverse reactions, allowing the system to reach equilibrium faster. However, it does not change the position of equilibrium, as it increases the rates of both reactions equally.
What happens if oxygen (O2) is removed from the reaction vessel?
-If oxygen (O2) is removed from the reaction vessel, the system will try to increase the concentration of O2 by shifting the reaction towards the reactants (to the left), which will increase the concentration and partial pressure of SO2 but decrease the concentration of SO3.
Outlines
🔬 Introduction to Le Chatelier's Principle
This section introduces Le Chatelier's Principle, which states that a system at equilibrium will react to a change by attempting to counteract it, essentially trying to maintain its equilibrium. An example is provided to illustrate how a system tries to adjust its state in response to changes such as pressure variations, maintaining the concept that any imposed change on a system at equilibrium will be met with an opposing reaction by the system to restore balance.
🧪 Applying Le Chatelier's Principle to Chemical Reactions
This paragraph dives into the application of Le Chatelier's Principle in the context of a chemical reaction involving reactants and products in different states (gaseous and liquid). It explores how changes in the concentration of a reactant or product influence the direction of the reaction shift (right or left) to counteract those changes, emphasizing the principle's role in predicting the outcome of such alterations in a system at equilibrium.
🔄 Effects of Catalysts and Reactant Changes
This segment explains how adding a catalyst or altering the amount of a reactant affects a chemical reaction's equilibrium. While catalysts speed up the reaction rate without affecting the equilibrium position, changes in reactant concentration can cause the reaction to shift in a direction that attempts to counteract the change, demonstrating the practical implications of Le Chatelier's Principle in chemical processes.
📈 Impact of Concentration and Pressure on Equilibrium
The discussion focuses on how changes in concentration and pressure influence the position of equilibrium in chemical reactions. It explains that altering the concentration of reactants or products can shift the equilibrium to either side, depending on whether the concentration is increased or decreased. Additionally, the effect of pressure changes due to volume adjustments on the system's equilibrium is explored, showing the interconnectedness of pressure, volume, and concentration changes.
🌡️ Adjusting Reaction Conditions: Volume and Pressure
This paragraph elaborates on the impact of changing the reaction container's volume on the equilibrium position, particularly for reactions involving gases. It explains the inverse relationship between pressure and volume and how an increase or decrease in volume can lead to a shift in the equilibrium towards the side with more or fewer moles of gas, respectively. The explanation helps understand how physical changes can influence chemical equilibria.
⚖️ Balancing Reactions: The Role of Partial Pressures
This final section ties together the concepts of partial pressures and their effect on the direction of equilibrium shifts. It discusses how changes in the partial pressure of reactants or products can cause the reaction to shift in a direction that attempts to restore equilibrium by compensating for the increased or decreased pressures, illustrating the detailed mechanisms through which Le Chatelier's Principle operates in gas-phase reactions.
Mindmap
Keywords
💡Le Chatelier's Principle
💡Dynamic Equilibrium
💡Reactants and Products
💡Shift in Equilibrium
💡Concentration
💡Pressure and Volume
💡Partial Pressure
💡Equilibrium Constant (K)
💡Catalyst
💡Inert Gases
Highlights
Le Chatelier's Principle is introduced as a fundamental concept in understanding how systems at equilibrium respond to changes.
A system at equilibrium will adjust to counteract any changes imposed on it, aiming to restore a balance.
In a chemical context, equilibrium is dynamic, with forward and reverse reactions occurring at equal rates, resulting in constant concentrations of products and reactants.
The addition of more reactant B to a system at equilibrium will cause the reaction to shift towards the products to reduce the concentration of B.
If the concentration of product C is increased, the system will shift in the direction that decreases the concentration of C, which is towards the left.
The state of a reactant (solid or liquid) does not affect the position of equilibrium, as their concentrations are not included in the equilibrium constant expression.
The addition of a catalyst speeds up the rate of both the forward and reverse reactions but does not change the position of equilibrium.
Removing hydrogen gas from the reaction vessel will cause the system to shift to the left to produce more hydrogen.
An increase in the concentration of a product, such as methane, will cause the system to shift left to decrease that product's concentration.
Increasing the partial pressure of hydrogen gas will cause the reaction to shift right, leading to a decrease in the concentration of carbon monoxide.
The introduction of an inert gas, like xenon, does not affect the position of equilibrium as it does not participate in the reaction.
If the concentration of oxygen is decreased, the system will try to increase it by shifting the reaction towards the reactants.
When the volume of the container is increased, the reaction will shift to the left to increase the total pressure by increasing the number of moles of gas.
Decreasing the volume of the container increases the total pressure, causing the reaction to shift to the right to decrease the number of moles of gas.
The ideal gas law (PV=nRT) is fundamental in understanding how changes in volume affect pressure and concentration in a gaseous reaction system.
The net effect of changing partial pressures of reactants and products is determined by the ratio of gas molecules on each side of the reaction.
The principles discussed apply to understanding and predicting the behavior of chemical reactions under various conditions of stress.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: