How to Use the Quadratic Formula in Chemistry
TLDRThe video script offers an insightful guide on the application of the quadratic formula in Chemistry, particularly in relation to ice tables and equilibrium problems. It begins by explaining the structure of a quadratic equation and the importance of arranging terms in descending order to solve for x. The script then illustrates the process of identifying coefficients and applying the quadratic formula, emphasizing the calculation of two x values and the selection of the positive value for concentration in chemistry problems. An example involving the decomposition of COBr2 gas is used to demonstrate the construction of an ICE table and the calculation of equilibrium concentrations using the equilibrium constant K. The video also addresses scenarios where both x values are positive, instructing viewers to choose the smaller value that does not exceed the initial concentration. The script concludes with a prompt to access practice problems and additional resources for further understanding.
Takeaways
- 🧪 The quadratic formula is essential for solving quadratic equations, which is useful in Chemistry 2 or AP Chemistry, especially when dealing with ICE tables.
- 📝 A quadratic equation is in the form of ax² + bx + c = 0, where a, b, and c are coefficients and x is the variable.
- 🔍 To apply the quadratic formula, ensure the equation is set to zero and in descending order of the variable's power.
- 📊 Identify the coefficients a, b, and c from the quadratic equation, including any negative signs.
- ➗ The quadratic formula provides two solutions for x, but in chemistry, only the positive value is considered valid for concentrations.
- 🧩 ICE tables are used to determine the concentrations of reactants and products at equilibrium in a chemical reaction.
- 🔄 Write the balanced chemical equation and initial concentrations (I), changes (C), and equilibrium concentrations (E) for each species in an ICE table.
- 📐 The equilibrium constant (K) expression only includes aqueous or gaseous species, not solids or liquids.
- 📉 If both x values from the quadratic formula are positive, choose the smaller value that is less than the initial concentration to avoid negative concentrations.
- 🧠 The quadratic formula can be used to solve for x in the equilibrium expression when dealing with complex chemical equilibrium problems.
- 📚 Practice problems and step-by-step video solutions are available to help understand when and how to use the quadratic formula in chemistry.
- ➡️ Always remember to check the validity of the x values obtained from the quadratic formula by ensuring they result in physically meaningful concentrations.
Q & A
What is the quadratic formula used for in the context of the provided transcript?
-The quadratic formula is used to solve for 'x' in a quadratic equation, which can be particularly helpful in Chemistry when dealing with concepts involving ice tables and equilibrium problems.
What is the generic form of a quadratic equation?
-The generic form of a quadratic equation is ax^2 + bx + c = 0, where 'a', 'b', and 'c' are coefficients, 'x' is the variable, and the powers are in descending order.
How do you identify the coefficients 'a', 'b', and 'c' in a quadratic equation?
-You identify the coefficients by looking at the quadratic equation in the form of ax^2 + bx + c = 0. The first coefficient is 'a', the second is 'b', and the last one is 'c'. Any negative signs in front of these coefficients should be included.
What is an ICE table and how is it used in Chemistry?
-An ICE table is a method used in Chemistry to systematically keep track of reactants and products in a chemical equilibrium. It stands for Initial, Change, and Equilibrium. It is used to write the balanced chemical equation, note the initial concentrations, predict the changes, and find the concentrations at equilibrium.
Why do we only use the positive value of 'x' when solving for concentrations at equilibrium in Chemistry?
-In Chemistry, concentrations cannot be negative, as they represent the amount of a substance present. Therefore, when using the quadratic formula and obtaining two x values, only the positive value is considered valid for representing concentrations at equilibrium.
What does the 'K' in an equilibrium expression represent?
-The 'K' in an equilibrium expression represents the equilibrium constant, which is a measure of the extent to which a chemical reaction proceeds to completion at a given temperature.
How do you determine which x value to use if both x values obtained from the quadratic formula are positive?
-If both x values are positive, you choose the x value that is smaller and less than the initial concentration given. This ensures that the calculated concentration at equilibrium is feasible and does not exceed the initial concentration.
What is the significance of the equilibrium constant expression in Chemistry?
-The equilibrium constant expression is significant in Chemistry as it provides a quantitative measure of the position of equilibrium for a reversible reaction. It allows chemists to predict the direction in which a reaction will proceed to establish equilibrium.
What is the order of operations when solving for 'x' using the quadratic formula?
-The order of operations when solving for 'x' using the quadratic formula is to first address exponents (since there are no parentheses with operations inside), followed by multiplication, addition, and then taking the square root of the result.
Why is it necessary to convert all terms to molarity when using an ICE table?
-It is necessary to convert all terms to molarity when using an ICE table because molarity is the standard unit for expressing the concentration of substances in a solution, which is essential for calculating and comparing equilibrium constants.
How does the quadratic formula help in finding the concentrations of chemical species at equilibrium?
-The quadratic formula helps in finding the concentrations of chemical species at equilibrium by solving for the variable 'x', which represents the change in concentration. This allows chemists to calculate the concentrations of reactants and products at the point of equilibrium.
What is the process of 'foiling' and when is it used in the context of the quadratic formula?
-Foiling is a method used for multiplying two binomials. It stands for 'First, Outer, Inner, Last', and it is used when multiplying terms in the quadratic formula, especially when dealing with expressions that require the expansion of squared terms or cross-multiplication of binomials.
Outlines
📚 Understanding the Quadratic Formula in Chemistry
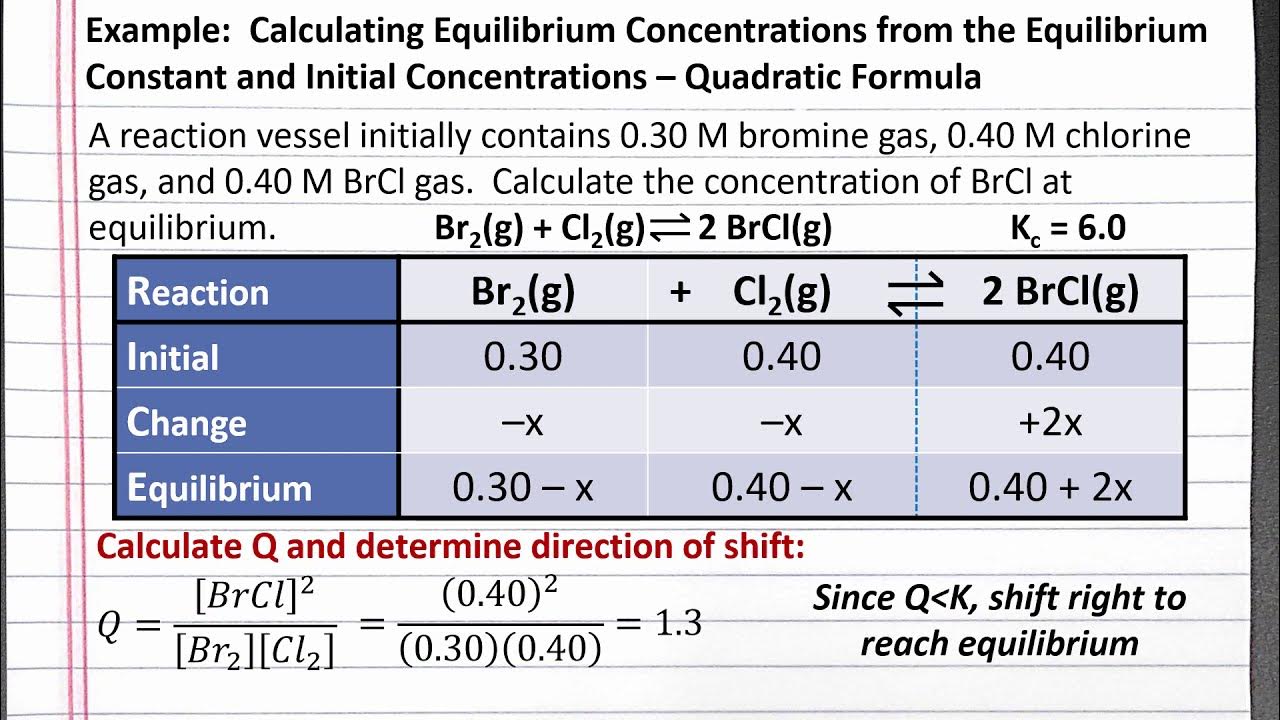
This paragraph introduces the importance of the quadratic formula in Chemistry, particularly when dealing with ICE tables. It explains the structure of a quadratic equation and how to identify the coefficients a, b, and c. The paragraph demonstrates how to apply the quadratic formula to solve for x, emphasizing the need for the equation to be in descending order and equal to zero. It also covers the calculation steps for the quadratic formula, leading to two possible x values. However, in chemistry, only the positive value is considered valid, as negative concentrations are not possible. An example of an equilibrium problem is provided to illustrate the application of the quadratic formula in chemistry.
🧪 Solving Quadratic Equations for Equilibrium Concentrations
The second paragraph delves into the process of using the quadratic formula to find equilibrium concentrations in chemistry problems. It outlines the steps to set up and solve a quadratic equation, including multiplying by the denominator, distributing values, and rearranging the equation into the proper form. The paragraph explains how to identify the coefficients a, b, and c for the quadratic formula and how to calculate the two possible x values. It emphasizes choosing the positive x value, as concentrations cannot be negative. The paragraph also addresses what to do when both x values are positive, instructing to select the smaller value that does not exceed the initial concentration. A detailed example is provided to illustrate these concepts, including building an ICE table, writing the equilibrium constant expression, and solving for equilibrium concentrations.
🔗 Further Practice with Quadratic Formula in Chemistry
The final paragraph encourages viewers to practice their skills with the quadratic formula by providing a link to additional resources, including practice problems and step-by-step video solutions. It offers tips and tricks for mastering the topic and invites viewers to return for more educational content after trying out the practice problems.
Mindmap
Keywords
💡Quadratic Formula
💡ICE Tables
💡Equilibrium Constant (K)
💡Coefficients
💡Molarity
💡Reactants and Products
💡Negative Concentration
💡Balanced Chemical Equation
💡Descartes' Rule of Signs
💡Order of Operations
Highlights
The quadratic formula is essential in Chemistry 2 or AP Chemistry for solving problems involving ice tables.
A quadratic equation is in the form of ax^2 + bx + c = 0, where a, b, and c are coefficients.
To solve for x in a quadratic equation, it must be set equal to zero and in descending order.
Identify coefficients a, b, and c from the quadratic equation before applying the quadratic formula.
When using the quadratic formula, ensure numbers are written in parentheses and follow the order of operations.
The quadratic formula yields two x values, but in chemistry, only the positive value is considered valid for concentrations.
In chemistry, the positive x value represents concentration, and negative concentrations are not possible.
An ICE table is used to determine the concentration of each species at equilibrium in a chemical reaction.
The initial concentration of reactants and products in an ICE table are labeled with I, changes with C, and equilibrium with E.
Equilibrium constant K is written with products raised to the power of their coefficients and reactants on the bottom.
Solids and liquids are not included in the equilibrium constant expression.
To use the quadratic formula, set the equilibrium expression equal to zero and rearrange terms.
When both x values from the quadratic formula are positive, choose the smaller value that is less than the initial concentration.
The quadratic formula can be necessary when solving for concentrations at equilibrium, especially when dealing with multiple reactants and products.
Practice problems with step-by-step video answers are available for further understanding of the topic.
The quadratic formula is a powerful tool for solving complex chemistry problems involving equilibrium and concentrations.
The sign of the coefficients in the quadratic formula must be included accurately to ensure correct calculations.
Concentrations at equilibrium are calculated using the equilibrium concentrations from the E row of the ICE table.
Transcripts
Browse More Related Video
CHEM 201: Calculating Equilibrium Concentrations from K and Initial Values – Quadratic Formula

Practice Problem: ICE Box Calculations
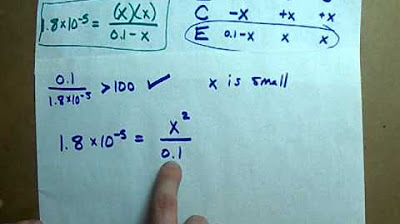
pH of a Weak Acid (0.1 M Acetic Acid) EXAMPLE

Equilibrium Problems #1

Equilibrium 2--Calculating Equilibrium

Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLE
5.0 / 5 (0 votes)
Thanks for rating: