Coulomb’s Law | Electronics Basics #2
TLDRIn this informative video, we delve into Coulomb's Law, a fundamental principle in physics that governs the interaction between electrically charged objects. Discovered by French physicist Charles Coulomb in 1785, this law is pivotal for understanding electromagnetism. It articulates that like charges repel and unlike charges attract, with the force's magnitude being directly proportional to the product of the charges and inversely proportional to the square of the distance between them. The video simplifies the concept with examples and introduces the superposition principle, demonstrating how to calculate forces in systems with multiple point charges. A must-watch for anyone interested in the basics of electromagnetism and electric forces.
Takeaways
- 📜 Coulomb's Law was first defined by French physicist Charles-Augustin de Coulomb in 1785 and is fundamental to the theory of electromagnetism.
- 🔋 The law describes the electrostatic force between two electrically charged objects, determining whether they attract or repel each other.
- 💥 Like charges repel (two positive or two negative charges) and unlike charges attract (one positive and one negative charge).
- ⚙️ Coulomb's Law applies to point charges, which are objects that can be considered as points if they are small compared to the distance between them.
- 📐 The force between the charges is along the straight line joining them, and the magnitude of this force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
- 📈 The equation for Coulomb's Law is F = (k * q1 * q2) / r^2, where F is the force in Newtons, k is the proportionality constant, q1 and q2 are the charges in Coulombs, and r is the distance between the charges in meters.
- 🌌 The value of the proportionality constant k depends on the medium between the objects and is approximately 9 * 10^9 N m^2/C^2 in a vacuum.
- 🔗 Coulomb's Law is similar to the universal law of gravitation in form, but they differ in that the electric force is much stronger than gravity and has different proportionality constants.
- 🔄 The superposition principle allows for the calculation of the total Coulomb force on a point charge from a system of point charges by summing the individual forces.
- 🔢 The electric force is a vector quantity with both magnitude and direction, which can be calculated based on the sign of the result.
- 🎥 The video also provides a practical example of calculating the force between two positive charges and one negative charge, demonstrating the application of the law.
Q & A
Who first defined Coulomb's Law and in what year?
-Coulomb's Law was first defined by French physicist Charles-Augustin de Coulomb in 1785.
What does Coulomb's Law describe?
-Coulomb's Law describes the interaction between electrically charged objects, quantifying the electrostatic force between them that causes attraction or repulsion.
What are the two possible interactions between two charged objects according to Coulomb's Law?
-Two charged objects can either repel each other (if they have the same charge) or attract each other (if they have opposite charges).
What is the condition for treating objects as point charges?
-Objects can be treated as point charges if they are very small compared to the distance between them.
How is the magnitude of the electric force between charged particles calculated?
-The magnitude of the electric force (F) is calculated using the equation F = k * (q1 * q2) / r^2, where q1 and q2 are the charges, r is the distance between them, and k is the proportionality constant.
What is the unit of electric force?
-The electric force is expressed in Newtons.
Why is the distance squared in the equation for electric force?
-The distance is squared because when the distance between objects doubles, the force between them reduces to a quarter of their original value.
What is the value of Coulomb's Law constant (k) in a vacuum?
-In a vacuum, the value of the Coulomb constant (k) is 1 divided by 4 pi epsilon naught (approximately 9 times 10 to the 9 Newton meters squared per Coulomb squared).
How does the electric force differ from the universal law of gravitation?
-While both involve forces, the electric force can be either attractive or repulsive depending on the charges, whereas the gravitational force is always attractive and depends on mass.
What is the superposition principle in the context of Coulomb's Law?
-The superposition principle states that for all linear forces, the total force is a vector sum of individual forces. This allows Coulomb's Law to be extended to include any number of point charges.
How can you determine the direction of the force between two charges?
-The direction of the force is determined by the line passing through both charges. A positive result in the calculation indicates repulsion, while a negative result indicates attraction.
Outlines
🔬 Introduction to Coulomb's Law and its Fundamental Concepts
This paragraph introduces Coulomb's Law, a fundamental principle in physics that describes the interaction between electrically charged objects. It explains that the law was first defined by French physicist Charles-Augustin de Coulomb in 1785 and was crucial for the development of electromagnetism theory. Coulomb's Law quantifies the electrostatic force between two charged objects, causing attraction or repulsion depending on whether the charges are like or unlike. The law applies to point charges, which are small relative to the distance between them, and states that the force between the charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The constant 'k' in the equation, known as the proportionality constant or Coulomb's constant, depends on the medium between the objects. The paragraph also compares Coulomb's Law to the universal law of gravitation, highlighting their similarities and differences.
📐 Calculation and Superposition Principle of Electrostatic Forces
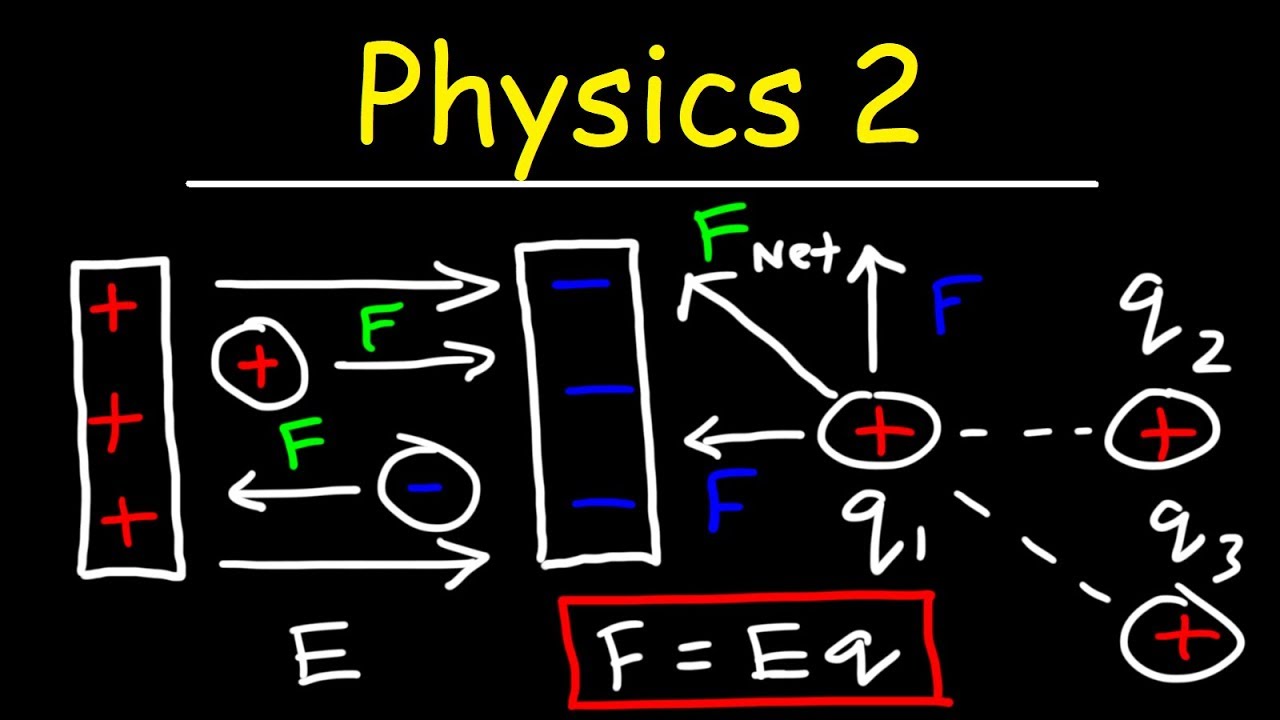
This paragraph delves into the practical application of Coulomb's Law by demonstrating how to calculate the force between two charges. It provides an example with specific values for charges and distance, leading to a calculation that results in a positive value, indicating repulsion. The paragraph further explains that if the charges were of opposite types, the result would be negative, indicating attraction. The concept of the superposition principle is introduced, stating that the total force on a point charge due to multiple point charges can be found by vector addition of the individual forces. An example with three point charges is used to illustrate how to calculate the total electric force, showing that the resultant force is the vector sum of the individual forces acting on a point charge.
Mindmap
Keywords
💡Coulomb's Law
💡Electrostatic Force
💡Point Charges
💡Electric Charge
💡Proportionality Constant (k)
💡Dielectric Constant (ε₀)
💡Vector Quantity
💡Superposition Principle
💡Electric Field
💡Charge Quantization
Highlights
Coulomb's Law was first defined by French physicist Charles Coulomb in 1785.
Coulomb's Law was essential to the development of the theory of electromagnetism.
The law describes the interaction between electrically charged objects and quantifies the electrostatic force between them.
Like charges repel each other, while unlike charges attract each other due to the electrostatic force.
Coulomb's Law applies to point charges, which are objects that can be treated as points if they are very small compared to the distance between them.
The electric force between two charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
The unit for electric force is the Newton, and the equation for Coulomb's Law is F = (k * q1 * q2) / r^2.
The proportionality constant k, also known as Coulomb's constant, depends on the medium where the objects reside.
In a vacuum, the value of the permittivity (epsilon naught) is approximately 8.854 × 10^-12 N m^2 C^-2.
Coulomb's Law and the universal law of gravitation share similarities but operate in completely different ways.
The electric force is much stronger than gravity and is determined by charge rather than mass.
Electrostatic forces can be either attractive or repulsive, unlike the gravitational force which is always attractive.
The force between two point charges is a vector quantity with both magnitude and direction.
The superposition principle allows for the calculation of the total force from multiple point charges acting on a single charge.
An example is given where two positive charges repel and a negative charge attracts a positive charge, demonstrating the calculation of forces using Coulomb's Law.
The video concludes with a mention of the next topic, the electric field, in the upcoming basic electronics video.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: