Lec-10 I Optical Activity in Lactic acid, tartaric acid I Applied Chemistry I Chemical Engineering
TLDRThe video lecture from Yoshi at Angie Institute of Engineering and Technology delves into the fundamentals of stereochemistry, exploring optical isomers, enantiomers, and diastereomers with examples. It explains the concept of plane polarized light and its significance in distinguishing between these isomers. The lecture also introduces the polarimeter, an instrument used to measure optical activity and differentiate between optical isomers based on their effect on polarized light, such as dextrorotatory and levorotatory compounds, and optically inactive ones.
Takeaways
- 📘 Applied Chemistry is the subject with the code 3130506, and the lecture series focuses on stereochemistry.
- 🔍 Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules, including optical isomers, enantiomers, and diastereomers.
- 🌟 Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other, like left and right hands.
- 🌟 Diastereomers are stereoisomers that are not mirror images of each other, resulting from different configurations at one or more chiral centers.
- 💡 Plane polarized light is light in which all the wave vibrations occur in a single plane, as opposed to non-polarized or unpolarized light where vibrations occur in multiple directions.
- 🔄 Polarization is the process of converting non-polarized light into polarized light, using a polarizer such as a prism or a Nicol prism.
- 🔍 Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image, often due to the presence of a chiral carbon atom bonded to four different groups.
- 📈 The plane of symmetry is an imaginary plane that divides an object into two mirror-image parts, which is a key concept in understanding chirality and optical activity.
- 🧪 Optical activity is the ability of certain molecules to rotate the plane of polarized light, and it is measured using an instrument called a polarimeter.
- 🌀 The rotation of plane polarized light can be either clockwise (dextrorotatory, denoted as + or d) or counterclockwise (levorotatory, denoted as - or l), indicating the compound's optical activity.
- 🧬 Lactic acid and tartaric acid are examples of compounds with chiral centers that exhibit optical activity, having different forms like dextrorotatory and levorotatory isomers.
Q & A
What is the subject code for Applied Chemistry in the lecture series?
-The subject code for Applied Chemistry is 3130506.
What is the significance of enantiomers in stereochemistry?
-Enantiomers are a type of optical isomers that are non-superimposable mirror images of each other. They are significant in stereochemistry because they can interact differently with plane polarized light, leading to different optical activities.
What are diastereomers and how do they relate to stereochemistry?
-Diastereomers are stereoisomers that are not mirror images of each other. They occur in molecules with multiple chiral centers when not all chiral centers have the same configuration. In stereochemistry, diastereomers can have different physical and chemical properties and can be separated based on their behavior towards plane polarized light.
What is plane polarized light and how does it differ from non-polarized light?
-Plane polarized light is light in which the vibrations occur in a single plane. This contrasts with non-polarized or unpolarized light, where the light waves vibrate in every direction. Plane polarized light is important in the study of optical isomers as it can be used to distinguish between different types of stereochemistry.
What is the process of converting non-polarized light to plane polarized light called?
-The process of converting non-polarized light to plane polarized light is known as polarization. This is typically achieved using a polarizer, which can be a prism or other specialized material that allows only light vibrating in a specific plane to pass through.
What is a plane of symmetry in the context of stereochemistry?
-A plane of symmetry in stereochemistry is a hypothetical plane that divides a molecule into two mirror-image halves. If a molecule has a plane of symmetry, it is not chiral and does not exhibit optical activity.
What is chirality and how is it related to optical activity?
-Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image. Molecules with chirality, particularly those with chiral carbon atoms, can exhibit optical activity, meaning they can rotate plane polarized light, which is a key concept in the study of stereochemistry.
How is optical activity measured in compounds?
-Optical activity in compounds is measured using an instrument called a polarimeter. This device allows the determination of how much a compound rotates plane polarized light, which is indicative of its optical activity.
What are the different types of optical rotation that can be observed with optically active compounds?
-Optically active compounds can exhibit either dextrorotatory (clockwise) or levorotatory (counterclockwise) rotation of plane polarized light. If there is no rotation, the compound is considered optically inactive.
How does the structure of lactic acid relate to its optical activity?
-Lactic acid contains a chiral carbon atom, making it a chiral molecule. It exists in two mirror-image forms, d-lactic acid and l-lactic acid, which are non-superimposable and exhibit different optical activities. The presence of the chiral carbon atom is what confers optical activity to lactic acid.
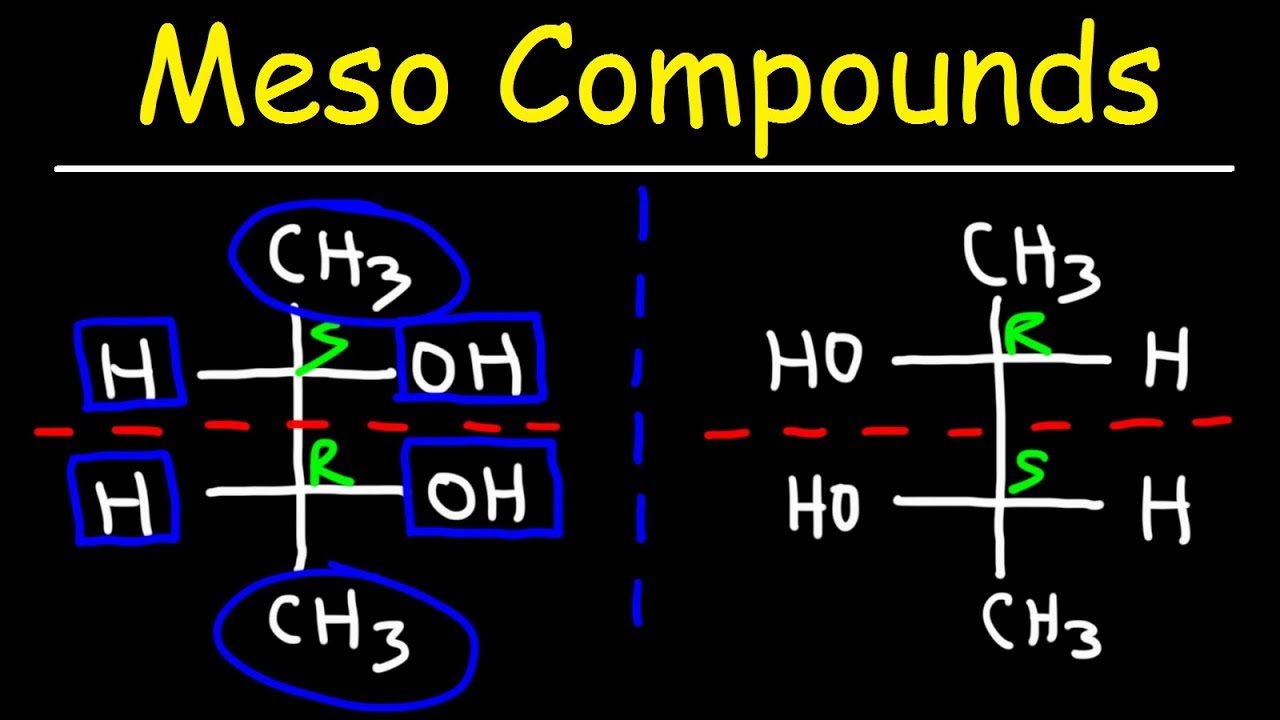
What are the four different forms of tartaric acid mentioned in the script?
-The four different forms of tartaric acid mentioned are dextrorotatory (d plus), levorotatory (l minus), meso (resonate), and a form that is optically inactive. These forms differ in the configuration of their chiral centers and their ability to rotate plane polarized light.
Outlines
🔬 Introduction to Stereochemistry and Polarized Light
Yoshi, from Angie Institute of Engineering and Technology, introduces the video lecture series on Applied Chemistry, focusing on stereochemistry, which is the study of how molecular structures differ in space. The lecture delves into the basics of stereochemistry, including the concepts of optical isomers, enantiomers, and diastereomers, using examples to illustrate these differences. A significant part of the lecture is dedicated to explaining plane polarized light, differentiating it from non-polarized light. Plane polarized light vibrates in a single plane, unlike non-polarized light, whose vibrations occur in multiple directions. The process of converting non-polarized light to plane polarized light, known as polarization, and the role of polarizers, such as prisms, are explained. The lecture emphasizes the importance of understanding plane polarized light to grasp the fundamental concepts of stereochemistry.
🌐 Plane of Symmetry, Chirality, and Optical Activity Measurement
This section explores further concepts essential to stereochemistry, such as the plane of symmetry and chirality. The plane of symmetry refers to a plane that divides a compound into two symmetrical parts. Chirality is introduced through the concept of a chiral carbon atom, which is bonded to four different substituents, making the compound optically active. Yoshi uses examples to clarify these concepts, highlighting how chiral compounds are non-superimposable mirror images of each other and thus are optically active. The lecture transitions to discussing how optical activity is measured using a polarimeter. The process involves converting unpolarized light to plane polarized light, which then passes through a sample. The polarimeter can detect the rotation of plane polarized light caused by optically active compounds, classifying them as dextrorotatory or levorotatory based on the direction of rotation.
🧪 Optical Activity in Compounds and Summary
In the concluding section, Yoshi demonstrates the concept of optical activity through the example of lactic acid, explaining how its chiral carbon leads to two forms: D and L lactic acid, which are mirror images of each other but non-superimposable. This principle extends to tartaric acid, which showcases multiple forms of optical activity, including dextrorotatory, levorotatory, and optically inactive forms. The lecture emphasizes understanding the structural basis of optical activity and its measurement, concluding with a demonstration of how polarimetry is used to distinguish between different optical isomers of compounds like lactic acid and tartaric acid. The comprehensive overview ties together the importance of stereochemistry in understanding the behavior of compounds in relation to polarized light.
Mindmap
Keywords
💡Stereochemistry
💡Optical Isomers
💡Enantiomers
💡Diastereomers
💡Plane Polarized Light
💡Polarizer
💡Chirality
💡Optically Active Compounds
💡Polarimeter
💡Plane of Symmetry
💡Dextrorotatory
💡Levorotatory
Highlights
Introduction to Applied Chemistry and subject code 3130506
Discussion on Stereochemistry and its basics
Explanation of optical isomers and enantiomers with examples
Definition and understanding of diastereomers
The concept of plane polarized light and its difference from non-polarized light
Process of polarization and the role of polarizers
Introduction to the plane of symmetry in compounds
Explanation of chirality and its significance in organic compounds
Identification of chiral carbon and its impact on optical activity
Measurement of optical activity using a polarimeter
Description of the working principle of a polarimeter
Differentiation between dextrorotatory and levorotatory compounds
Explanation of optically inactive compounds
Observation of optical activity in lactic acid
Discussion on the structures of d and l lactic acid, and their mirror images
Explanation of different forms of optical isomers in tartaric acid
Understanding of the meso form and its optical inactivity
Transcripts
Browse More Related Video

5.7 Optical Activity | Organic Chemistry

Lec-09 I Optical Isomers I Applied Chemistry I Chemical engineering

More Stereochemical Relationships: Crash Course Organic Chemistry #9

5.1 Overview of Isomers | Constitutional Isomers and Stereoisomers | Organic Chemistry
Meso Compounds

[H2 Chemistry] 2021 Topic 9 Isomerism
5.0 / 5 (0 votes)
Thanks for rating: