5.7 Optical Activity | Organic Chemistry
TLDRThe video script delves into the fascinating world of optical activity, a phenomenon related to isomers and stereochemistry. It explains how plane polarized light interacts with chiral compounds, causing it to rotate in one of two directions, distinguishing optically active compounds from optically inactive ones. The concept of enantiomers and their ability to rotate light in opposite directions is explored, as well as the importance of the specific rotation, a characteristic value for each pair of enantiomers. The script also covers the calculation of specific rotation, taking into account concentration and path length, and introduces the terms optical purity and enantiomeric excess. By providing an example with S2 bromobutane, the video illustrates how to determine the composition of a mixture of enantiomers based on observed specific rotation. The lesson concludes with the significance of optical purity and enantiomeric excess in understanding the composition and optical activity of chiral compound mixtures.
Takeaways
- 🌟 Optical activity is a phenomenon where plane polarized light is rotated by chiral compounds, and different enantiomers rotate light in opposite directions.
- 🔍 A polarizing filter is used to create plane polarized light from unpolarized light by blocking out certain orientations of light's electric field.
- ⚖️ Chiral compounds are optically active, meaning they can rotate plane polarized light, whereas achiral compounds and racemic mixtures are optically inactive.
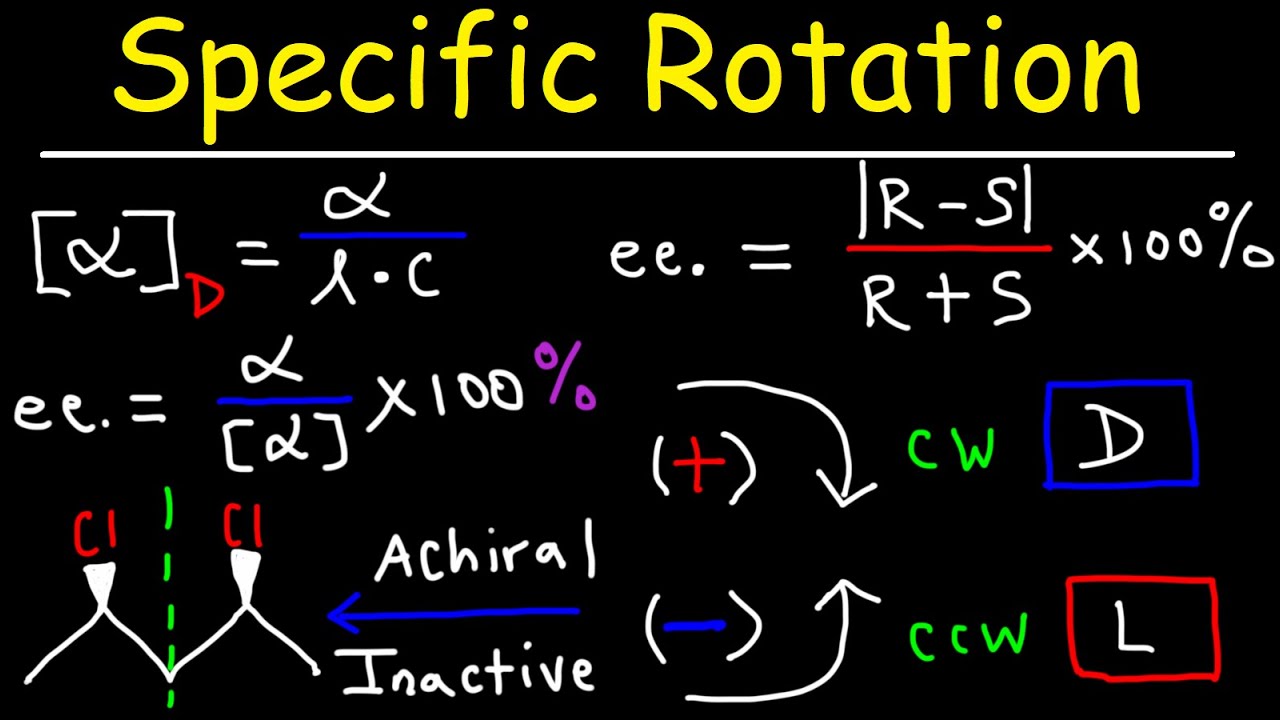
- 📊 The specific rotation is a characteristic value for a pair of enantiomers, measured in degrees and is dependent on the concentration of the compound and the path length the light travels through.
- 📐 The calculation of specific rotation requires the concentration to be in grams per milliliter and the path length in decimeters.
- 🔬 Optical purity and enantiomeric excess are measures of the presence of one enantiomer over the other in a mixture and are calculated based on the observed specific rotation.
- 🧬 Relative configurations (d or l, plus or minus) describe the direction of light rotation by enantiomers but cannot be determined by molecular structure alone.
- 🔬 Absolute configurations (R or S) can be assigned by examining the structure of a molecule using the Cahn-Ingold-Prelog priority rules.
- ⚖️ A racemic mixture, which is a 50/50 mixture of enantiomers, is optically inactive because the rotations of the two enantiomers cancel each other out.
- 📚 The specific rotation of a compound can be used to determine the optical purity and enantiomeric excess of a mixture, which are always equal to each other.
- 📉 If a mixture is not exactly 50/50, it will be optically active, and the degree of rotation can be used to calculate the composition of the mixture.
Q & A
What is optical activity?
-Optical activity refers to the ability of certain compounds, known as chiral compounds, to rotate plane polarized light either to the right (dextrorotatory, labeled as 'd' or '+') or to the left (levorotatory, labeled as 'l' or '-'). This rotation is used to distinguish between different enantiomers of a chiral compound.
How does a polarizing filter work?
-A polarizing filter works by blocking light waves that are not aligned in a specific orientation. It allows only light waves vibrating in a particular plane to pass through, thus converting unpolarized light into plane polarized light.
What is the difference between absolute configuration and relative configuration?
-Absolute configuration refers to the three-dimensional arrangement of atoms in a chiral molecule, which can be designated as 'R' or 'S' using the Cahn-Ingold-Prelog priority rules. Relative configuration, on the other hand, describes how a compound rotates plane polarized light, either to the right ('d' or '+') or to the left ('l' or '-'), and does not directly provide information about the molecular structure.
Why can't you determine the relative configuration (d or l) by looking at the molecular structure?
-The relative configuration (d or l) cannot be determined by looking at the molecular structure alone because it involves the interaction of the compound with plane polarized light, which requires experimental measurement using a polarimeter. There is no direct correlation between the 'R/S' system and the 'd/l' system.
What is a racemic mixture?
-A racemic mixture is a 50:50 mixture of both enantiomers of a chiral compound. Because the two enantiomers rotate plane polarized light in opposite directions, the mixture is optically inactive, meaning it does not rotate the plane of polarized light.
How is specific rotation calculated?
-Specific rotation is calculated using the formula: Specific Rotation = (Observed Rotation in degrees) / (Concentration in g/mL × Path length in dm). It is a characteristic value for a pair of enantiomers and provides information about the extent to which a compound rotates plane polarized light under specific conditions.
What are the units required for the calculation of specific rotation?
-The units required for the calculation of specific rotation are grams per milliliter (g/mL) for the concentration and decimeters (dm) for the path length.
What is optical purity?
-Optical purity is a measure of the extent to which a mixture of enantiomers is not racemic, indicating the presence of one enantiomer over the other. It is calculated as the observed specific rotation divided by the specific rotation of one pure enantiomer and is expressed as a percentage.
What is the relationship between optical purity and enantiomeric excess?
-Optical purity and enantiomeric excess are equivalent measures. They both represent the percentage difference between the amounts of the two enantiomers in a mixture, indicating how much more of one enantiomer is present compared to the other.
How can you determine the composition of a mixture of enantiomers based on the observed specific rotation?
-You can determine the composition of a mixture of enantiomers by comparing the observed specific rotation with the known specific rotations of the pure enantiomers. Using the optical purity, you can then calculate the percentage of each enantiomer present in the mixture, provided you know the specific rotation of at least one pure enantiomer.
Why might optical activity be de-emphasized in an undergraduate organic chemistry course?
-Optical activity might be de-emphasized in an undergraduate organic chemistry course because it involves a level of complexity and specialized knowledge that may not be central to the broader curriculum. Additionally, it may not be as commonly encountered in practical applications or exams compared to other topics in organic chemistry.
Outlines
🌟 Introduction to Optical Activity in Stereochemistry
This paragraph introduces the topic of optical activity, a phenomenon related to isomers and stereochemistry. It discusses the potential for this topic to be de-emphasized in undergraduate courses but emphasizes its importance for those studying organic chemistry. The speaker, Chad, welcomes viewers to his chemistry preparation channel, where he aims to make science understandable and enjoyable. He introduces a new organic chemistry playlist that will be updated weekly throughout the 2020-21 school year and encourages viewers to subscribe and enable notifications to stay updated. The paragraph also touches on plane polarized light, the rotation of light by chiral compounds, and the concept of optical activity in relation to enantiomers and racemic mixtures.
🔍 Understanding Relative Configurations and Specific Rotation
The second paragraph delves into the concept of relative configurations, which are related to how plane polarized light is rotated by enantiomers. It explains the terms dextro (d or +) for right rotation and levo (l or -) for left rotation, and clarifies that these cannot be determined by molecular structure alone but require a polarimeter for measurement. The paragraph also discusses the lack of a direct correlation between absolute configurations (R and S) and relative configurations (d or l), and how knowing one enantiomer's rotation allows the inference of the other's. The specific rotation, a characteristic property of enantiomers, is introduced, along with its calculation considering concentration and path length, using unusual units like grams per milliliter and decimeters.
🧪 Optical Purity and Enantiomeric Excess Calculations
This paragraph focuses on optical purity and enantiomeric excess, explaining what happens when a mixture of enantiomers is not exactly 50:50, leading to optical activity. An example using S2 bromobutane is provided to illustrate how to calculate the composition of a mixture based on observed specific rotation. The specific rotation values for pure enantiomers of S2 bromobutane are given, and the optical purity is calculated by comparing the observed rotation with that of pure R2 bromobutane. The calculation shows that the optical purity equals the enantiomeric excess, which in this case is 50%, indicating there is 50% more of the R enantiomer than the S enantiomer in the mixture.
📊 Visualizing Enantiomeric Composition in a Racemic Mixture
The final paragraph provides a visual explanation of the composition of a mixture of enantiomers, using the example of a racemic mixture where the specific rotations of R and S enantiomers cancel each other out. It breaks down how a mixture with 75% R and 25% S enantiomers results in an optically active solution with half the rotation of pure R. The explanation helps to visualize why the observed specific rotation is negative 11.5 degrees, reflecting the excess of R enantiomer. The paragraph concludes with an invitation for viewers to like, share, and ask questions in the comments section, and to check out study guides and practice problems on the speaker's website.
Mindmap
Keywords
💡Optical Activity
💡Plane Polarized Light
💡Chiral Compounds
💡Enantiomers
💡Racemic Mixture
💡Relative Configurations
💡Absolute Configurations
💡Specific Rotation
💡Optical Purity
💡Enantiomeric Excess
💡Polarimeter
Highlights
Optical activity is a key topic in the study of isomers and stereochemistry.
Plane polarized light has an orientation, and its interaction with chiral compounds can result in rotation.
Chiral compounds are optically active, meaning they can rotate plane polarized light, while achiral compounds are optically inactive.
Enantiomers of a chiral compound rotate plane polarized light in opposite directions.
A racemic mixture, which is a 50/50 mixture of enantiomers, is optically inactive on average.
Relative configurations (d or l, plus or minus) refer to the direction of light rotation and are determined using a polarimeter.
There is no direct correlation between absolute configurations (R and S) and relative configurations (d or l) without experimental data.
Specific rotation is a characteristic value for a pair of enantiomers and depends on concentration and path length.
The specific rotation calculation requires concentration in grams per milliliter and path length in decimeters.
Optical purity and enantiomeric excess are measures of the presence of one enantiomer over the other in a mixture.
A mixture with a non-racemic composition can be optically active, and its specific rotation can be used to determine the composition.
The optical purity and enantiomeric excess are equal and indicate the percentage of one enantiomer over the other.
An example calculation demonstrates how to determine the composition of a mixture based on observed specific rotation.
The specific rotation of a pure enantiomer can be used to infer the composition of a mixture with a known observed rotation.
The relationship between the percentages of enantiomers in a mixture can be solved using linear combination or elimination methods.
The optical activity of a mixture is influenced by the relative amounts and specific rotations of the enantiomers present.
Understanding optical activity and specific rotation is crucial for the analysis of chiral compounds in organic chemistry.
The lesson provides a comprehensive overview of optical activity, calculations, and the practical implications for enantiomer composition.
Transcripts
Browse More Related Video
Optical Activity - Specific Rotation & Enantiomeric Excess - Stereochemistry Youtube

Lec-10 I Optical Activity in Lactic acid, tartaric acid I Applied Chemistry I Chemical Engineering
Meso Compounds

5.1 Overview of Isomers | Constitutional Isomers and Stereoisomers | Organic Chemistry
Organic Chemistry 1 Exam 2 Review

More Stereochemical Relationships: Crash Course Organic Chemistry #9
5.0 / 5 (0 votes)
Thanks for rating: