Meso Compounds
TLDRThis script delves into the concept of chirality in molecules, explaining the difference between enantiomers and meso compounds. It clarifies that while both contain chiral centers, meso compounds have an internal line of symmetry, making them optically inactive and not rotating plane polarized light. The script guides viewers through identifying optical activity by looking for symmetry, using examples to illustrate how to determine if a molecule is a meso compound or an enantiomer, thus being optically active or inactive.
Takeaways
- 🧪 The script discusses the concept of chirality in molecules, specifically focusing on enantiomers and meso compounds.
- 🔍 It explains that a molecule's mirror image can be an enantiomer if it is not superimposable with the original, indicating a chiral molecule.
- 🤔 The script clarifies that meso compounds, despite having chiral centers, are not enantiomers because they possess an internal line of symmetry.
- 🌀 It highlights that meso compounds are optically inactive due to their symmetry, meaning they do not rotate plane polarized light.
- 🔄 The process of determining the configuration of chiral centers using the Cahn-Ingold-Prelog priority rules is described, including the R and S notation.
- 🔄 The script emphasizes the importance of reversing the order of groups when analyzing chiral centers to determine their configuration.
- 📚 It provides examples to illustrate the difference between optically active and optically inactive molecules, using the presence or absence of an internal line of symmetry as a key factor.
- 📉 The script challenges viewers to identify whether given molecules are optically active or inactive, based on their structural symmetry.
- 📝 It offers a step-by-step approach to analyzing molecular structures for optical activity, including identifying chiral centers and lines of symmetry.
- 📚 The transcript includes a variety of examples to help viewers practice distinguishing between meso and chiral compounds.
- 🔬 The script concludes by reinforcing the concept that meso compounds, despite their chiral centers, are optically inactive due to their symmetrical nature.
Q & A
What is the significance of a molecule's mirror image in the context of chirality?
-The mirror image of a molecule helps determine if it is an enantiomer or a meso compound. Enantiomers are non-superimposable mirror images that are chiral and can rotate plane polarized light, while meso compounds, despite having chiral centers, are symmetrical and do not rotate plane polarized light due to their internal line of symmetry.
What is the difference between enantiomers and meso compounds?
-Enantiomers are chiral molecules that are mirror images of each other and are not identical. They can rotate plane polarized light. Meso compounds, on the other hand, are chiral molecules with chiral centers but possess an internal line of symmetry, making them identical to their mirror images and optically inactive.
How can you determine the configuration of a chiral center using the Cahn-Ingold-Prelog (CIP) priority rules?
-You assign priorities to the groups attached to the chiral center, starting with the highest priority (usually OH > CH3 > H). Then, following the rule, if the sequence is clockwise, it's R, and if it's counterclockwise, it's S.
Why are meso compounds optically inactive?
-Meso compounds are optically inactive because they have a line of symmetry, making them identical to their mirror images. This symmetry negates the ability to rotate plane polarized light, which is a characteristic of optical activity.
What does it mean for a molecule to be optically active?
-A molecule is optically active if it can rotate plane polarized light. This property is associated with chiral molecules that do not have an internal line of symmetry, allowing them to exist as enantiomers.
How can you identify if a molecule has an internal line of symmetry?
-You can identify an internal line of symmetry by observing if the molecule can be divided into two identical halves. If the halves are mirror images of each other, the molecule has an internal line of symmetry.
What is the practical implication of a molecule being optically active or inactive?
-Optically active molecules can interact with light in a way that alters its plane of polarization, which is important in various applications such as the study of molecular structures and in the pharmaceutical industry where the effectiveness of a drug can depend on its stereochemistry.
Can all chiral molecules be meso compounds?
-No, not all chiral molecules can be meso compounds. Only those chiral molecules that have an internal line of symmetry and are identical to their mirror images can be classified as meso compounds.
How does the presence of a line of symmetry affect the optical activity of a molecule?
-The presence of a line of symmetry in a molecule means it is a meso compound, which is optically inactive. The symmetry causes the molecule to be identical to its mirror image, thus it does not rotate plane polarized light.
What is the importance of understanding the difference between enantiomers and meso compounds in chemistry?
-Understanding the difference between enantiomers and meso compounds is crucial in chemistry as it impacts the physical and chemical properties of molecules, including their reactivity, polarity, and interactions with other molecules, which can have significant implications in various chemical and biological processes.
Outlines
🧪 Understanding Chirality and Meso Compounds
This paragraph discusses the concept of chirality in molecules, focusing on the identification of meso compounds and enantiomers. It explains that meso compounds, despite having chiral centers, are optically inactive due to their internal symmetry. The speaker guides the audience through the process of determining the configuration of chiral centers using the Cahn-Ingold-Prelog priority rules and emphasizes the importance of recognizing the presence or absence of an internal line of symmetry to distinguish between optically active and inactive molecules.
🌟 Differentiating Optical Activity in Molecular Structures
The second paragraph continues the theme of molecular chirality, providing examples to illustrate how to determine whether a molecule is optically active or inactive. It reiterates that the presence of an internal line of symmetry indicates a meso compound, which is optically inactive, while the absence of such symmetry suggests an optically active compound. The paragraph walks through several molecular examples, pointing out the differences in their structures that lead to their classification as either meso or enantiomers, and encourages the audience to practice identifying these properties in various molecules.
Mindmap
Keywords
💡Compound
💡Mirror Image
💡Enantiomer
💡Meso Compound
💡Chiral Centers
💡Configuration
💡Optically Active
💡Optically Inactive
💡Line of Symmetry
💡Stereoisomerism
💡Plane-Polarized Light
Highlights
Explaining the concept of chiral centers and their configurations in molecules.
Differentiating between enantiomers and meso compounds in molecular structures.
The process of drawing the mirror image of a molecule and its implications on chirality.
Understanding that chiral centers can change configurations when mirror images are drawn.
The significance of the internal line of symmetry in determining if a molecule is meso or chiral.
Meso compounds are optically inactive due to their symmetry, while chiral molecules are optically active.
The method of assigning priorities to groups in chiral centers to determine configuration (R or S).
The importance of reversing the order when analyzing chiral centers to determine their configuration.
How the presence or absence of an internal line of symmetry affects the optical activity of a molecule.
Examples of molecules with and without internal lines of symmetry and their optical activity.
The concept that meso compounds, despite having chiral centers, are symmetrical and thus optically inactive.
The practical application of determining optical activity in various molecular structures.
The challenge of identifying optically active and inactive molecules through problem-solving exercises.
The role of molecular symmetry in classifying a molecule as a meso compound or a chiral molecule.
How the orientation of atoms in 3D space affects the identification of chiral and meso compounds.
The importance of recognizing that not all molecules with chiral centers are optically active.
The final example illustrating the distinction between optically active and inactive molecules based on symmetry.
Transcripts
Browse More Related Video

Stereochemistry: Meso Compounds, Diastereomers

Chiral vs Achiral Molecules - Chirality Carbon Centers, Stereoisomers, Enantiomers, & Meso Compounds

5.7 Optical Activity | Organic Chemistry
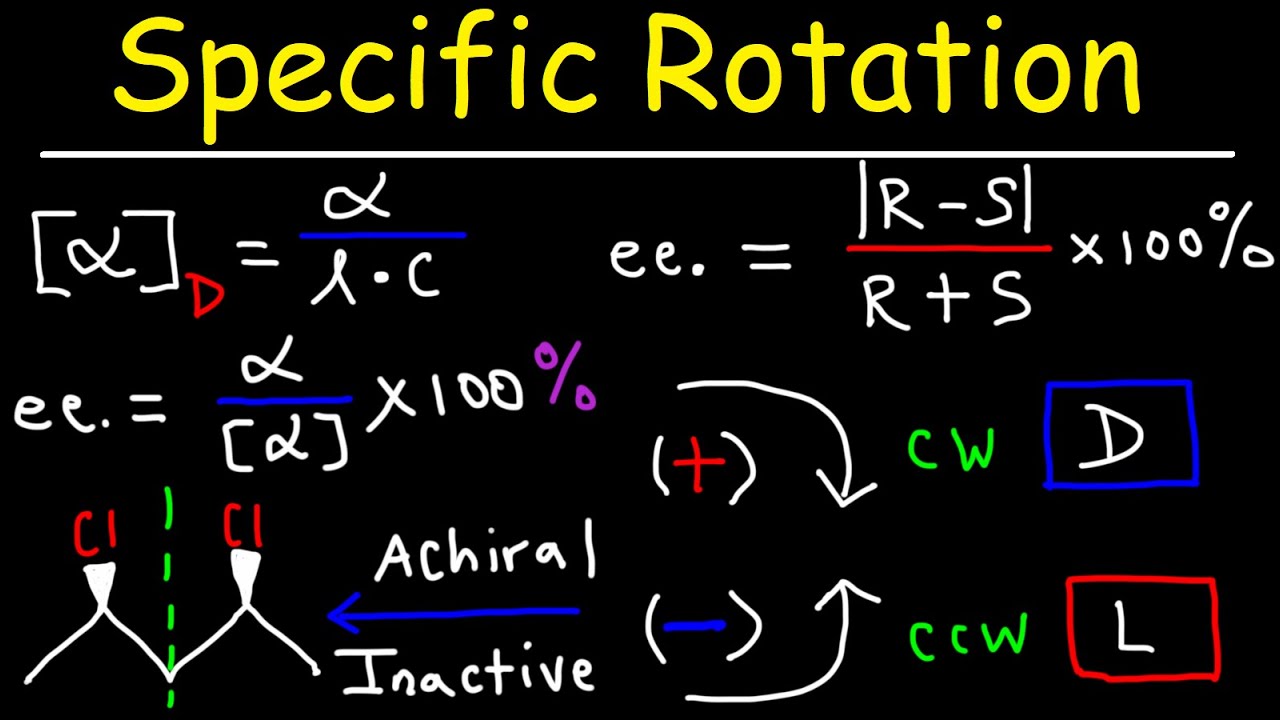
Optical Activity - Specific Rotation & Enantiomeric Excess - Stereochemistry Youtube

Lec-10 I Optical Activity in Lactic acid, tartaric acid I Applied Chemistry I Chemical Engineering

Stereoisomers, Enantiomers, Meso Compounds, Diastereomers, Constitutional Isomers, Cis & Trans
5.0 / 5 (0 votes)
Thanks for rating: