AP Chemistry Unit 8 Review: Acids and Bases
TLDRThis video delves into the concepts of acids and bases, focusing on the Arrhenius and Brønsted-Lowry definitions, and explains the process of neutralization. It covers the pH scale, the relationship between pH and pOH, and introduces the topic of strong and weak acids and bases. The video also discusses the behavior of water as an amphoteric substance and the concept of buffer solutions, which resist changes in pH. Additionally, it touches on the practical application of titration and the use of the Henderson-Hasselbalch equation. The content is presented in an engaging and informative manner, suitable for viewers seeking to understand the fundamentals of acid-base chemistry.
Takeaways
- 📚 The basic definition of acids and bases according to Arrhenius is that acids release H+ ions (protons) and bases release OH- ions (hydroxide).
- 🧪 Neutralization reactions occur when an acid reacts with a base, resulting in the formation of water (H2O) and a salt from the remaining ions.
- 📈 The pH scale is a measure of acidity or basicity, with values less than 7 indicating acidic solutions, 7 being neutral, and values greater than 7 indicating basic solutions.
- 🔄 The relationship between pH and pOH is such that pH + pOH = 14, where pOH is the negative logarithm of the hydroxide ion concentration.
- 🧬 The concept of conjugate acids and bases is introduced by the Bronsted-Lowry theory, where a base is a substance that can accept a proton (H+) and an acid is a substance that can donate a proton.
- 🌊 Water is amphoteric, meaning it can act as both an acid and a base, depending on the reaction conditions.
- 🔋 Strong acids and bases fully dissociate in solution, while weak acids and bases only partially dissociate, retaining some of their original form.
- 📊 The strength of an acid or base can be determined by its dissociation constant (Ka for acids, Kb for bases), with larger values indicating stronger acids or bases.
- 🏋️♂️ Buffer solutions resist changes in pH when small amounts of acids or bases are added, due to the presence of a weak acid and its conjugate base or a weak base and its conjugate acid.
- 📝 The Henderson-Hasselbalch equation (pKa + log([A-]/[HA]) = pH) is a useful tool for calculating pH in buffer solutions and understanding the behavior of weak acids and bases.
- 🧪 Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration, with the pH changing during the process providing important information about the reaction.
Q & A
What is the basic definition of an acid according to the Arrhenius theory?
-According to the Arrhenius theory, an acid is a substance that dissociates in water to yield hydrogen ions (H+).
How do you define a base in the context of the Arrhenius theory?
-In the Arrhenius theory, a base is a substance that dissociates in water to yield hydroxide ions (OH-).
What is a neutralization reaction and what products does it yield?
-A neutralization reaction is a chemical reaction in which an acid and a base react to form water (H2O) and a salt. The salt is a compound made up of the cation from the base and the anion from the acid.
What is the pH scale and how does it relate to the acidity or basicity of a solution?
-The pH scale is a numerical scale that ranges from 0 to 14 and is used to specify the acidity or basicity of a solution. A pH less than 7 indicates an acidic solution, a pH of 7 is neutral, and a pH greater than 7 indicates a basic solution.
How can you calculate the pH of a solution given the concentration of hydrogen ions?
-The pH of a solution can be calculated using the formula pH = -log[H+], where [H+] represents the concentration of hydrogen ions in the solution, expressed in moles per liter.
What is the relationship between pH and pOH, and what is their sum at 25 degrees Celsius?
-pH and pOH are related by the equation pH + pOH = 14 at 25 degrees Celsius. This relationship holds because it is based on the water autoionization constant (Kw), which is equal to 1 x 10^-14 at this temperature.
What is the difference between strong and weak acids?
-Strong acids are those that completely dissociate in water, releasing all their hydrogen ions. Weak acids, on the other hand, only partially dissociate, holding onto some of their hydrogen ions.
How can you determine if a salt is acidic, basic, or neutral?
-A salt is determined to be acidic, basic, or neutral based on the strength of the parent acid and base from which its ions are derived. If the salt is formed from a strong acid and a weak base, it will be acidic. If it's from a weak acid and a strong base, it will be basic. If both the acid and base are strong or both are weak, the salt will be neutral.
What is the concept of buffer solutions and how do they work?
-Buffer solutions are solutions that resist significant changes in pH when small amounts of an acid or a base are added. They work by having a weak acid and its conjugate base (or a weak base and its conjugate acid) present in the solution, which helps to neutralize added acids or bases and maintain a stable pH.
What is the Henderson-Hasselbalch equation and how is it used?
-The Henderson-Hasselbalch equation is a useful tool for calculating the pH of a solution containing a weak acid and its conjugate base. The equation is pKa + log([A-]/[HA]) = pH, where pKa is the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
How is the concentration of a strong acid determined during a titration with a strong base?
-During a titration of a strong acid with a strong base, the concentration of the strong acid can be determined by adding the base until the pH reaches 7 (neutral point). The volume of the base used, multiplied by its concentration, will give you the moles of the base added, which will equal the moles of the acid that was neutralized.
Outlines
📚 Introduction to Acids and Bases
The video begins with an apology for the delay in releasing the educational content and introduces the topic of acids and bases, which is part of the AP Chemistry curriculum. The instructor, Cara, explains that due to the pandemic, certain units including acids and bases were not covered in her previous course. Despite the challenges, she has managed to create a video to cover this important topic, motivated by the positive feedback and requests from her audience. Cara emphasizes the importance of understanding the basic concepts of acids and bases and promises to delve into the details of these chemical substances.
🧪 The Arrhenius Definition of Acids and Bases
Cara introduces the Arrhenius definition of acids and bases, explaining that acids are substances that produce hydrogen ions (H+) and bases are those that produce hydroxide ions (OH-). She also mentions the concept of hydronium ions (H3O+) and their interchangeability with H+ in discussions of acid-base reactions. The video then moves on to discuss the concept of neutralization reactions, where an acid and a base react to form water (H2O) and a salt. Cara emphasizes the importance of understanding the Arrhenius definition and provides examples to illustrate how to calculate pH values based on the concentration of H+ ions in a solution.
📈 pH and PoH Calculations
This section delves into the mathematical aspect of acid-base chemistry, focusing on the calculation of pH and PoH values. Cara explains the relationship between the concentration of hydrogen ions and pH, using the negative logarithm function. She provides examples to illustrate how to convert between these values and introduces the concept of PoH, which is related to the concentration of hydroxide ions. The video emphasizes the inverse relationship between pH and PoH, culminating in the important takeaway that their sum equals 14 at standard conditions.
🧬 Practical Examples of pH Calculations
Cara presents practical examples to demonstrate the calculation of pH values for solutions with given amounts of acids or bases. She uses sulfuric acid (H2SO4) and sodium hydroxide (NaOH) to illustrate how to find the molarity of hydrogen ions and hydroxide ions in a solution and subsequently calculate the pH. The video also touches on the concept of strong and weak acids and bases, providing a basic understanding of how their strength affects the pH of solutions. Cara encourages viewers to practice these calculations to become comfortable with the process.
🌿 Introducing the Bronsted-Lowry Definition
The video introduces an alternative definition of acids and bases known as the Bronsted-Lowry theory. According to this definition, acids are proton (H+) donors, and bases are proton acceptors. Cara explains this concept with examples of reactions between ammonia (NH3) and hydrochloric acid (HCl), highlighting the formation of conjugate acid-base pairs. The video also discusses the amphoteric nature of water, which can act as both an acid and a base depending on the reaction conditions.
🔋 Strong and Weak Acids and Bases
Cara differentiates between strong and weak acids and bases, providing examples and mnemonic devices to help remember which substances fall into each category. She explains that strong acids fully dissociate in water, releasing all their hydrogen ions, while weak acids only partially dissociate. The video also covers strong bases, typically hydroxides of alkali metals and some alkaline earth metals, and contrasts them with weak bases like ammonia. Cara emphasizes the importance of understanding these distinctions for solving problems in AP Chemistry.
🧪 Equilibrium and the Common Ion Effect
This section focuses on the equilibrium of weak acids and bases in solution, introducing the concept of the equilibrium constant (Ka and Kb). Cara explains how to calculate these constants and how they relate to the strength of an acid or base. The video also discusses the common ion effect, which influences the position of equilibrium in a solution containing a common ion. Cara uses the example of water dissociation (Kw) to illustrate how changes in temperature can affect the equilibrium constant and the pH of neutral solutions.
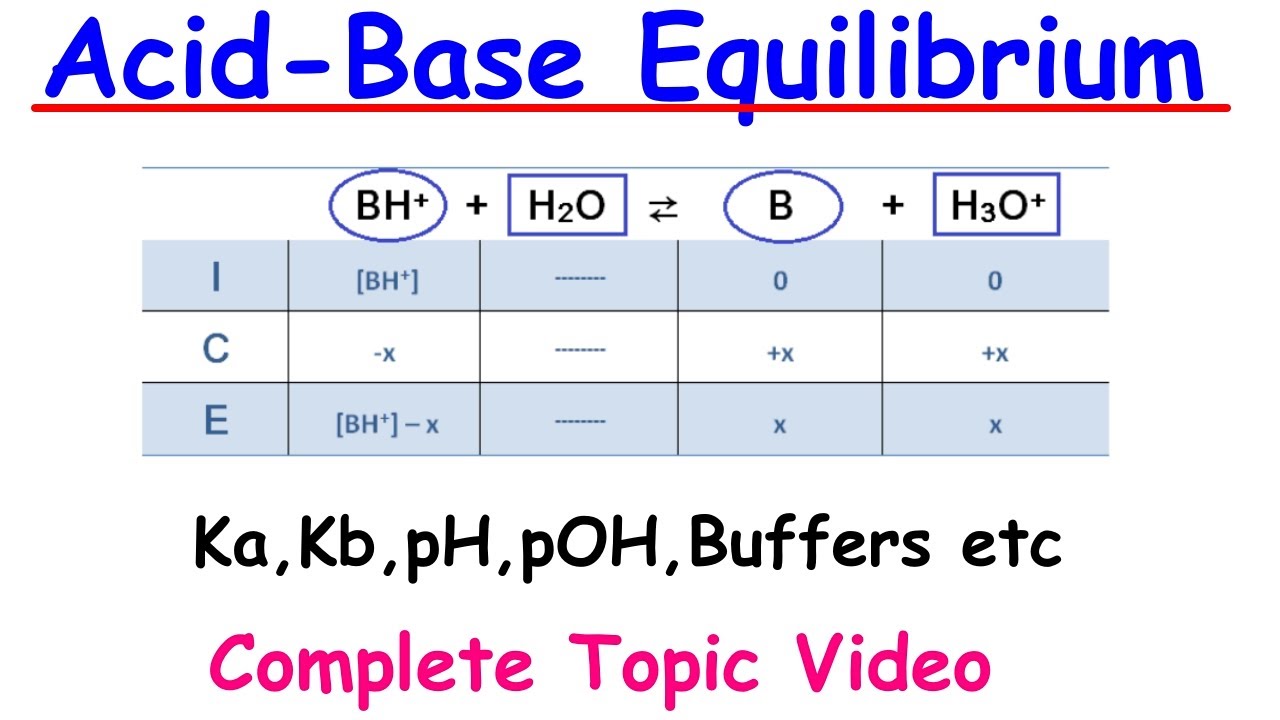
📊 ICE Charts and Weak Acid Titrations
Cara introduces the use of ICE (Initial, Change, Equilibrium) charts for visualizing and calculating the concentrations of species in solution at equilibrium. She demonstrates how to use these charts for weak acids and their titrations with strong bases, providing a step-by-step example using nitrous acid (HNO2). The video explains how to set up the ICE chart, make assumptions to simplify calculations, and ultimately determine the pH of the resulting solution. Cara also briefly touches on the concept of buffers and their role in resisting changes in pH.
📈 The Henderson-Hasselbalch Equation
Cara presents the Henderson-Hasselbalch equation, a useful tool for simplifying the calculation of pH in buffer solutions. She explains how this equation relates the pH of a solution to the pKa of a weak acid and the concentrations of the acid and its conjugate base. The video provides an example of how to use the Henderson-Hasselbalch equation to find the pH of a solution containing a weak acid and its salt, demonstrating the practical application of this important concept in acid-base chemistry.
🧪 Titration of Weak Acids with Strong Bases
The video concludes with a discussion on the titration of weak acids with strong bases. Cara explains the characteristic curve of such a titration and how to interpret it. She provides an example using nitrous acid (HNO2) and sodium hydroxide (NaOH), illustrating how to calculate the pH at various stages of the titration. The video emphasizes the buffer effect of the weak acid at the beginning of the titration and how the pH changes as the strong base converts more of the weak acid into its conjugate base. Cara concludes by summarizing the key points covered in the video and encourages viewers to practice these concepts for their AP Chemistry studies.
Mindmap
Keywords
💡Acids and Bases
💡pH
💡Neutralization Reaction
💡Arrhenius Theory
💡Hydronium Ion (H3O+)
💡Salt
💡pOH
💡Bronsted-Lowry Theory
💡Conjugate Acid-Base Pairs
💡Amphoteric
💡Strong and Weak Acids and Bases
💡Titration
Highlights
Introduction to the video's topic: acids and bases unit 8 in AP Chemistry.
Explanation of the basic definition of acids and bases according to the Arrhenius theory.
Discussion on the neutralization reactions between acids and bases, resulting in water and a salt.
Clarification that H+ ions can also be represented as H3O+ ions, which are essentially the same thing.
Elucidation of the net ionic equation for all neutralization reactions, demonstrating their similarity.
Explanation of the pH scale and its relation to the acidity or basicity of a solution.
Presentation of the formula for calculating pH, which is the negative logarithm of the H+ ion concentration.
Introduction to the concept of PoH and its relationship with pH, summing up to 14.
Practical examples of calculating pH and PoH using given hydrogen ion concentrations.
Discussion on the difference between strong and weak acids and bases, with examples provided.
Explanation of the Bronsted-Lowry definition of acids and bases, which expands on the Arrhenius theory.
Illustration of acid-base reactions according to the Bronsted-Lowry theory, including the concept of conjugate acids and bases.
Introduction to the concept of amphoteric substances, such as water, which can act as both acids and bases.
Explanation of the equilibrium constant (Kw) for water dissociation and its value at standard temperature.
Discussion on how the value of Kw can affect the pH of a neutral solution at different temperatures.
Introduction to the concept of buffer solutions and their role in resisting pH changes.
Explanation of the Henderson-Hasselbalch equation and its utility in simplifying pH calculations.
Demonstration of how to perform titrations with strong acids and bases, as well as weak acids with strong bases.
Conclusion and encouragement for viewers to engage with the content and seek further information.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: