[H2 Chemistry] 2022 Topic 14 Acid-Base equilibria 1
TLDRThis chemistry lesson delves into acid-base equilibrium, building on foundational concepts from JC1. It emphasizes the ICE table for problem-solving and introduces the Bronsted-Lowry theory, explaining acid-base reactions through proton transfer. The lecture covers weak acid ionization, calculating pH, and buffer solutions, highlighting the importance of understanding assumptions made during calculations. It also explores salt hydrolysis, discussing its impact on solution pH and the role of weak acids, bases, and cations with high charge density in hydrolysis reactions.
Takeaways
- 📚 The lecture builds upon the understanding of chemical equilibrium and solubility products, focusing on acid-base equilibrium and the ICE table as a useful tool for problem-solving.
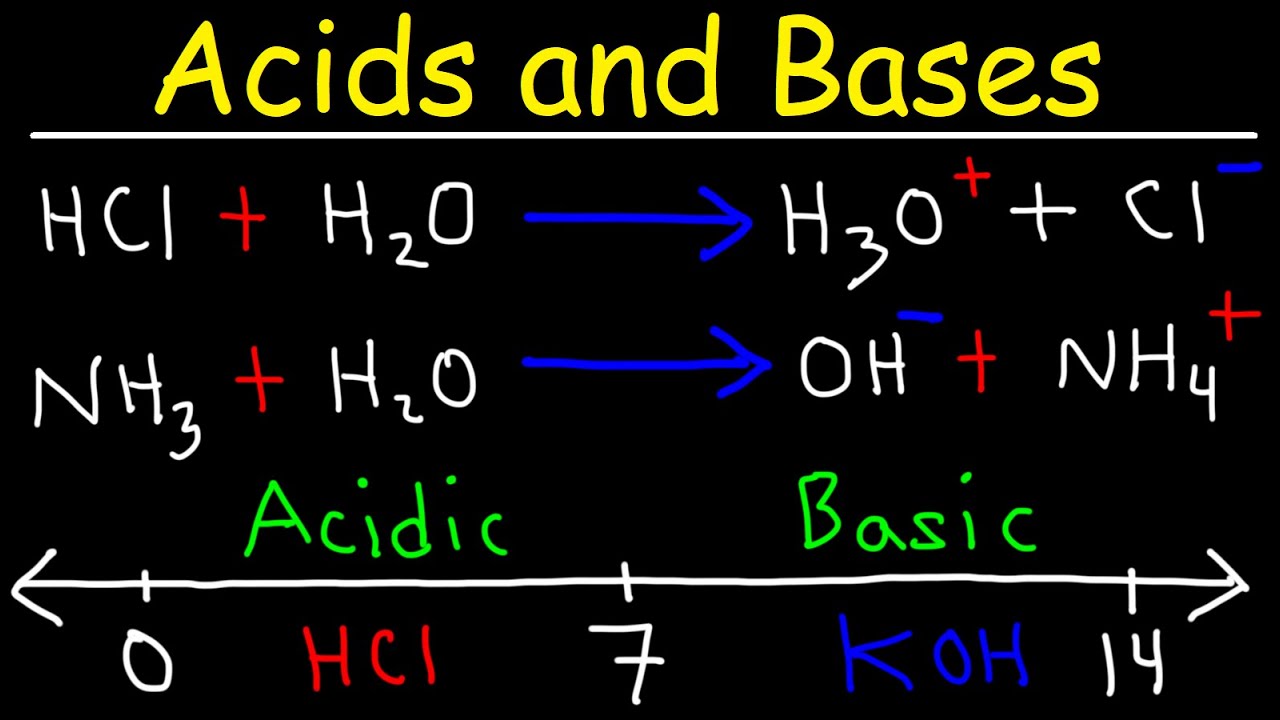
- 🔍 The Bronsted-Lowry theory is central to understanding acids as proton donors and bases as proton acceptors, with the transfer of a proton being the key reaction in acid-base chemistry.
- 🌡 The strength of an acid or base is not directly related to its concentration, but rather to its ability to ionize or dissociate in water, with strong acids and bases doing so completely.
- 🔄 The concept of conjugate acid-base pairs is introduced, highlighting that a substance can act as either an acid or a base depending on the reaction context.
- 🧪 The ICE table is emphasized as a method to visualize and calculate equilibrium concentrations in acid-base reactions, although it may become less necessary as familiarity with the concepts grows.
- 📉 The importance of understanding the equilibrium constant (Ka for acids and Kb for bases) and their relationship with pH and pOH is discussed, as these constants guide the position of equilibrium.
- 📚 The autoionization of water and its temperature dependence are explained, noting that an increase in temperature leads to an increase in the ionization of water and a corresponding increase in the value of Kw.
- 🔢 The calculation of pH in solutions of strong and weak acids and bases is covered, with special attention to the approximations made for weak acids when the concentration of H+ is less than 10^-7 M.
- ⚗️ The impact of salt hydrolysis on the pH of solutions is introduced, explaining how the reaction of ions with water can lead to either acidic or basic solutions depending on the strength of the parent acid or base.
- 🛠️ The practical application of understanding acid-base equilibrium is highlighted in areas such as buffer solutions, titration curves, and pH calculations, which are essential for advanced chemistry studies.
Q & A
What is the main focus of topic 14 on acid-base equilibrium?
-The main focus of topic 14 is to understand the concepts of acid-base equilibrium, including the ICE table method for solving problems, the Bronsted-Lowry theory of acids and bases, and the process of ionization or dissociation of weak acids and bases in water.
What is the Bronsted-Lowry definition of an acid and a base?
-According to the Bronsted-Lowry theory, an acid is a proton (H+) donor, and a base is a proton (H+) acceptor.
Why is the ICE table method useful in acid-base equilibrium problems?
-The ICE table method is useful because it helps to organize and simplify the process of solving equilibrium problems by systematically keeping track of the initial concentrations, changes in concentrations, and equilibrium concentrations of the species involved in the reaction.
What is the relationship between the acid dissociation constant (Ka) and the strength of an acid?
-The larger the Ka value, the stronger the acid, as it indicates a greater extent of ionization. Conversely, a smaller Ka value indicates a weaker acid.
What is the significance of the term 'conjugate acid-base pair' in acid-base chemistry?
-A conjugate acid-base pair refers to the products formed when an acid donates a proton (becomes the conjugate base) and a base accepts a proton (becomes the conjugate acid). These pairs differ by a proton (H+).
How does the strength of an acid relate to its concentration?
-The strength of an acid is not directly related to its concentration. A weak acid can be concentrated but still only partially ionizes, whereas a strong acid will ionize completely even if it is dilute.
What is the autoionization of water, and how does it relate to the pH and pOH of a solution?
-Autoionization of water is the slight ionization of water into hydrogen ions (H+) and hydroxide ions (OH-). The pH and pOH of a solution at 25°C are related through the equation pH + pOH = 14, which is derived from the ionic product of water (Kw = [H+][OH-] = 1x10^-14 at 25°C).
Why is it incorrect to simply add the autoionization contribution of water to the concentration of H+ from a strong acid in a solution?
-It is incorrect because the presence of H+ from a strong acid affects the autoionization equilibrium of water, slightly suppressing it. Therefore, the actual concentration of H+ in the solution will be slightly less than the sum of the contributions from the acid and the autoionization of water.
What is the concept of 'salt hydrolysis' and why does it occur?
-Salt hydrolysis occurs when a salt containing a weak acid or base ion reacts with water, leading to the formation of H3O+ or OH- ions, making the solution acidic or basic, respectively. It occurs due to the reaction of the weakly ionizing salt with water, which can polarize and stabilize the ions, leading to a shift in the equilibrium position.
How can you determine if a salt solution will be acidic, alkaline, or neutral?
-You can determine the pH of a salt solution by considering the strength of the acid and base from which the salt is derived. If the salt is from a strong acid and strong base, the solution will be neutral. If from a strong acid and a weak base, or a weak acid and a strong base, the solution will be acidic or alkaline, respectively, depending on which is the weaker component.
Outlines
📚 Introduction to Acid-Base Equilibrium
The script introduces Topic 14 on acid-base equilibrium, which is a continuation of chemical equilibrium concepts previously learned. The ICE table is emphasized as a useful tool for solving problems, despite not always being necessary. The instructor advises students to recall the Bronsted-Lowry theory of acids and bases, where acids are proton donors and bases are proton acceptors. The partial ionization of ethanoic acid in water is discussed, highlighting the formation of ethanoids and H3O+ ions, illustrating the concept of conjugate acid-base pairs and the arbitrary nature of these terms. The lecture aims to deepen the understanding of acid-base reactions and their equilibrium states.
🔍 Deep Dive into Acid-Base Reactions and Conjugate Pairs
This paragraph delves deeper into the identification of acids and bases in reactions, focusing on the transformation of these substances into their conjugate pairs upon ionization. The instructor uses the reaction between ethanoic acid and water to illustrate this process, explaining how the position of equilibrium depends on the strength of the acids and bases involved. The concept of conjugate acid-base pairs is further clarified, with examples provided to help students distinguish between strong and weak acids and bases. The importance of understanding these distinctions is emphasized in relation to the equilibrium position and the Ka values of acids.
📝 Exercise Walkthrough: Identifying Conjugate Acid-Base Pairs
The script presents an exercise that challenges students to identify conjugate acid-base pairs in various reactions. It discusses the reactions of nitric acid with ammonia and ammonia with water, explaining how the roles of acids and bases can change depending on the reaction partner. The importance of perspective in identifying conjugate acids and bases is highlighted, with a reminder that these roles are not absolute but relative to the specific reaction being considered.
🌡️ Temperature Effects on Acid-Base Equilibrium
The effects of temperature on the autoionization of water and the resulting pH are discussed. The instructor explains that the autoionization of water is an endothermic process, leading to an increase in the concentration of H+ and OH- ions as temperature rises. The concept of neutrality at different temperatures is explored, noting that while the pH of pure water may change with temperature, the solution remains neutral as long as the concentrations of H+ and OH- ions are equal. The instructor also clarifies misconceptions regarding the pH of water at temperatures above 25 degrees Celsius.
🧪 Calculations of pH in Acid and Base Solutions
The script provides a step-by-step guide to calculating the pH of strong and weak acids and bases. It emphasizes the need to consider the autoionization of water when the concentration of the acid or base is less than 10^-7 M. The pH of a solution of a strong acid (HI) and a strong base (Ba(OH)2) are calculated, demonstrating the process of determining the concentration of H+ ions and subsequently the pH. The importance of understanding the difference between concentrated and dilute solutions in the context of acid and base strength is highlighted.
🔄 Understanding Hydrolysis and Its Effects on pH
This paragraph introduces the concept of salt hydrolysis, explaining how it occurs when salts derived from weak acids or bases react with water. The instructor discusses how the hydrolysis of these salts can lead to either acidic or alkaline solutions, depending on the strength of the acid or base from which the salt is derived. The effects of high charge density cations on hydrolysis are also explored, with examples provided to illustrate how these cations can lead to acidic solutions due to the production of H3O+ ions.
📉 The Impact of Dilution on Acid Strength
The script examines how dilution affects the strength of acids, using the example of ethanoic acid. It explains that as the concentration of the acid decreases, the pH increases, but the degree of ionization also increases. This observation leads to a discussion on the reliability of pH and degree of dissociation as indicators of acid strength, noting that these indicators can change with concentration and are not always accurate measures of strength.
🧪 Hydrolysis Reactions of Salts and Their Solutions' pH
The script discusses the hydrolysis reactions of various salts and their effects on solution pH. It explains that salts derived from strong acids and bases will result in neutral solutions, while salts from weak acids or bases will lead to acidic or alkaline solutions. The instructor provides examples of different salts, including sodium carbonate and ammonium chloride, and explains the hydrolysis reactions that occur with these salts, resulting in either acidic or alkaline solutions.
📚 Conclusion of Section Three and Preview of Buffer Solutions
The script concludes the discussion on salt hydrolysis and sets the stage for the next section on buffer solutions. It summarizes the key points covered in the section, including the impact of salt hydrolysis on solution pH and the factors that influence the extent of hydrolysis. The instructor also provides a brief overview of what will be covered in the upcoming section, inviting students to stay tuned for more in-depth exploration of buffer solutions.
Mindmap
Keywords
💡Acid-Base Equilibrium
💡ICE Table
💡Bronsted-Lowry Theory
💡Conjugate Acid-Base Pairs
💡pH
💡Autoionization of Water
💡Ka and Kb
💡Titration
💡Buffer Solutions
💡Salt Hydrolysis
Highlights
Introduction to Topic 14 on acid-base equilibrium, emphasizing the importance of ICE tables for problem-solving.
Explanation of the Bronsted-Lowry theory defining acids as proton donors and bases as proton acceptors.
Discussion on the partial ionization of weak acids like ethanoic acid in water, forming conjugate bases.
Clarification that the terms 'dissociation' and 'ionization' are used interchangeably in acid-base scenarios.
Illustration of the ethanoic acid ionization process using arrow pushing to show the transfer of protons.
Introduction of the concept of conjugate acid-base pairs and their role in maintaining equilibrium.
Differentiation between strong and weak acids and bases, with examples and the significance of their ionization in water.
The arbitrary nature of labeling acids and bases based on the direction of the reaction.
Importance of recognizing the strength of acids and bases in determining the position of equilibrium.
Introduction of the acid dissociation constant (Ka) and its role in quantifying the strength of acids.
Explanation of the relationship between pH, pOH, and the ionic product of water (Kw).
Endothermic nature of water's autoionization and its temperature dependence.
Calculation of pH in strong acid and base solutions, incorporating the autoionization of water.
Exercise solutions demonstrating the calculation of pH for various acid-base systems.
Differentiation between concentrated and dilute solutions of acids and their impact on pH calculations.
Introduction to buffer solutions and their importance in maintaining a stable pH.
Explanation of salt hydrolysis and its impact on the pH of solutions containing salts derived from weak acids or bases.
Discussion on the hydrolysis of metal cations with high charge density and its resulting acidic solutions.
Practical applications of understanding acid-base equilibrium in inorganic qualitative analysis.
Concluding remarks summarizing the key points of the acid-base equilibrium topic and setting the stage for the next section on buffer solutions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: