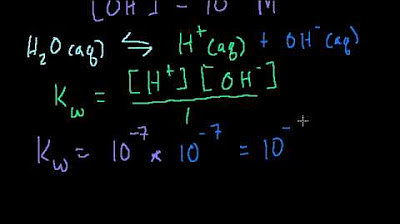What is Kw (The Ion Product Constant of Water)
TLDRThe video script explains the concept of the ion product constant for water, known as kW, which is the equilibrium constant for the dissociation of water into H+ and OH- ions. It emphasizes that kW is a special constant with a value of 1.0 x 10^-14 at 25°C, relating to the neutral pH of water. The script also discusses how temperature affects kW, with higher temperatures leading to an increased value due to more energetic molecular collisions and greater ion dissociation. Understanding kW is crucial for calculating ion concentrations in solutions.
Takeaways
- 💧 The concept of kW is introduced as the ion product constant for water, representing the equilibrium constant for the dissociation of liquid water into H+ and OH- ions.
- 🔄 The equilibrium process of water dissociating into ions and ions recombining to form water is continuous and always happening in any solution.
- 📝 The equilibrium constant (kW) is expressed as the product of the concentrations of the ions (H+ and OH-), without including the liquid water in the expression.
- 🌡️ The value of kW at 25 degrees Celsius is 1.0 x 10^-14, which is related to the pH of water being exactly 7 at this temperature.
- 🔢 Given the concentration of either H+ or OH-, one can calculate the concentration of the other using the equation kW = [H+][OH-], by rearranging and solving for the unknown ion concentration.
- 🌞 The value of kW is affected by temperature, with higher temperatures leading to an increase in the constant due to more energetic molecular collisions and greater ion dissociation.
- 📉 At lower temperatures, the equilibrium shifts to favor the recombination of ions into water molecules, resulting in a lower value of kW.
- 📚 Understanding the relationship between temperature and kW is important, especially when dealing with solutions where the concentrations of H+ or OH- are known or need to be calculated.
- 🎓 The script emphasizes the importance of knowing how to interconvert between the concentrations of H+ and OH- using the constant kW in various chemical and environmental contexts.
- 🌟 The video script serves as a comprehensive guide to the concept of kW, its significance in aqueous solutions, and its dependency on temperature, providing a solid foundation for further studies in chemistry.
Q & A
What is the ion product constant for water, also known as?
-The ion product constant for water is known as the equilibrium constant, denoted as kW.
What process does the kW constant represent?
-The kW constant represents the equilibrium process where liquid water dissociates into H+ and OH- ions.
Is the dissociation of water into ions a reversible process?
-Yes, the dissociation of water into H+ and OH- ions is a reversible process, with some ions recombining to form water molecules.
How is the equilibrium constant (kW) expressed in terms of the concentrations of H+ and OH- ions?
-The equilibrium constant kW is expressed as the product of the concentrations of H+ and OH- ions, with the formula kW = [H+][OH-].
What is the value of kW at 25 degrees Celsius?
-At 25 degrees Celsius, the value of kW is 1.0 × 10^-14.
How is the value of kW related to the pH of water?
-The value of kW is related to the pH of water in that at 25 degrees Celsius, the pH is exactly 7, which is half of the value of kW (1.0 × 10^-14).
What happens to kW when the temperature increases?
-As the temperature increases, the equilibrium shifts to the right, resulting in a higher value of kW due to more energetic collisions and greater dissociation of water into ions.
At 60 degrees Celsius, how does the value of kW compare to its value at 25 degrees Celsius?
-At 60 degrees Celsius, the value of kW is higher than its value at 25 degrees Celsius, due to the increased temperature promoting more dissociation of water molecules.
What can you do if you are given the concentration of either H+ or OH- in a solution?
-If you are given the concentration of either H+ or OH-, you can use the equation kW = [H+][OH-] to calculate the concentration of the other ion by rearranging and solving for it.
How does the equilibrium of water dissociation change at lower temperatures?
-At lower temperatures, the equilibrium shifts to the left, resulting in a lower value of kW because the molecules move more slowly, allowing positive and negative ions to recombine more easily.
What is the approximate value of kW at 0 degrees Celsius?
-At 0 degrees Celsius, the value of kW is approximately 1/6 of what it is at 25 degrees Celsius, indicating a significant decrease in the dissociation of water into ions.
Outlines
📚 Introduction to the Ion Product Constant of Water (kW)
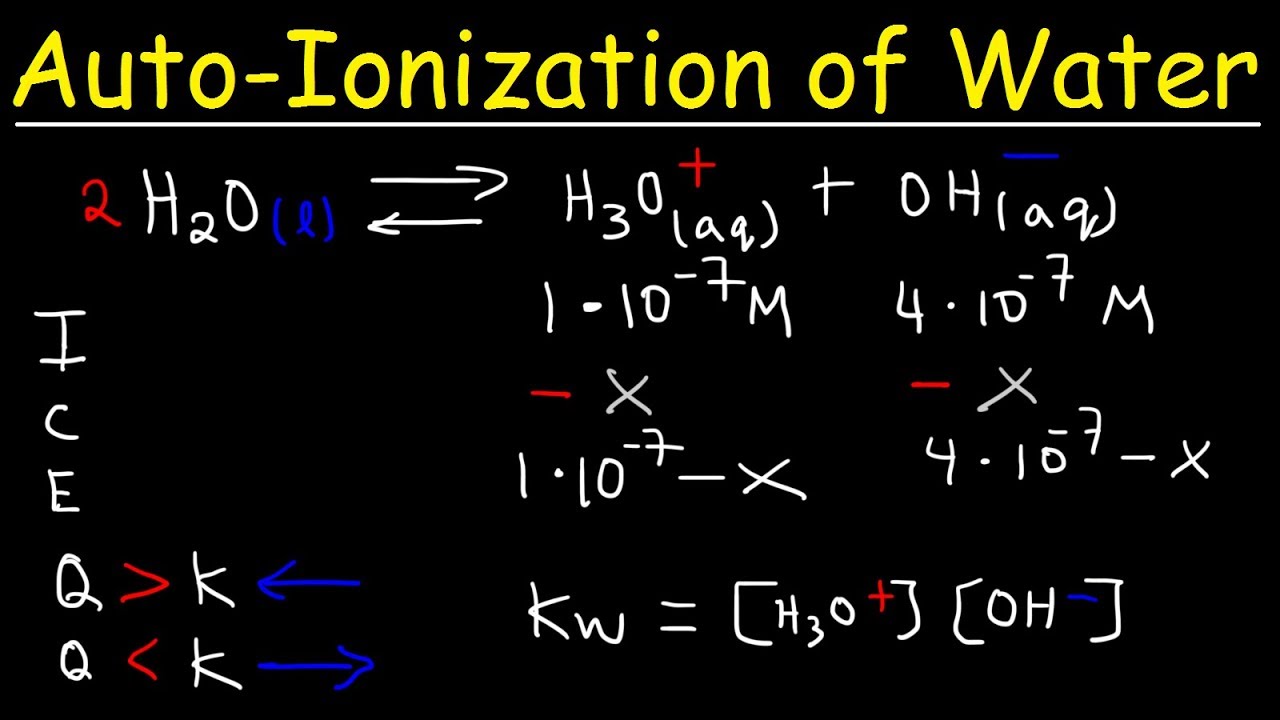
This paragraph introduces the concept of the ion product constant for water, known as kW. It explains that kW represents the equilibrium constant for the dissociation of liquid water into H+ and OH- ions, a process that occurs continuously in any solution. The script clarifies that while some may depict the equilibrium with two water molecules forming an H3O+ ion and an OH- ion, the standard expression involves only H+ and OH- ions. It also highlights the unique nature of kW, emphasizing that at 25 degrees Celsius, its value is 1.0 x 10^-14, which is directly related to the pH of water being 7. The paragraph further explains how to use kW to calculate the concentration of OH- if the concentration of H+ is given, and vice versa, by rearranging the equilibrium expression.
🌡️ The Effect of Temperature on the Ion Product Constant (kW)
This paragraph delves into the impact of temperature on the value of kW. It clarifies that kW is temperature-dependent and varies with changes in temperature due to its equilibrium nature. The script explains that at higher temperatures, the equilibrium shifts to the right, leading to an increased dissociation of water into ions, which results in a higher kW value. Conversely, at lower temperatures, the equilibrium shifts to the left, and the ions have a higher chance of recombining, leading to a lower kW value. The example provided indicates that at 0 degrees Celsius, kW is significantly lower than at 25 degrees Celsius. The paragraph concludes by encouraging viewers to explore the mathematical relationship between temperature and the equilibrium constant in more detail through additional resources.
Mindmap
Keywords
💡kW
💡Equilibrium
💡Hydrogen ions (H+)
💡Hydroxide ions (OH-)
💡Equilibrium constant
💡pH
💡Temperature
💡Dissociation
💡Concentration
💡Reversible process
💡Ions
Highlights
kW is the ion product constant for water.
It represents the equilibrium constant for the dissociation of liquid water into H+ and OH- ions.
The process of water dissociation is reversible and continuously happening in any solution.
At equilibrium, water can also form H3O+ and OH- through the collision of two water molecules.
The equilibrium constant expression does not include liquids, only the aqueous concentrations of H+ and OH- ions.
At 25 degrees Celsius, the value of kW is 1.0 x 10^-14.
The pH of water at 25 degrees Celsius is 7, which is related to the value of kW.
kW can be used to calculate the concentration of OH- if the concentration of H+ is known, and vice versa.
The value of kW is affected by temperature, as it is an equilibrium constant.
At higher temperatures, the equilibrium shifts to the right, increasing the value of kW.
At 60 degrees Celsius, the value of kW is higher than 1.0 x 10^-14.
At lower temperatures, the equilibrium shifts to the left, resulting in a lower value of kW.
At 0 degrees Celsius, kW is significantly lower, closer to 1/6 of its value at 25 degrees Celsius.
Higher temperatures lead to a higher kW due to more energetic collisions and increased ion dissociation.
For a deeper understanding of how temperature affects equilibrium constants, refer to the video on how the equilibrium constant K changes with temperature.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: