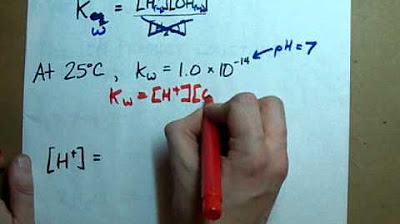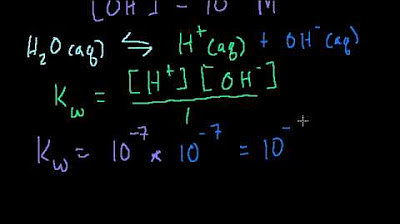The Ionic Product of Water Kw
TLDRThis video script delves into the ionic product of water, known as the ionic product constant (kW), highlighting its significance in determining the acidity or alkalinity of aqueous solutions. It explains that all aqueous solutions contain H+ and OH- ions from the partial dissociation of water, with the equilibrium constant (kW) being affected by temperature. The script demonstrates how the pH of pure water at 298 K is 7, and how increasing temperature causes an increase in kW, resulting in a decrease in pH, yet maintaining neutrality since the concentrations of H+ and OH- ions remain equal.
Takeaways
- 🌊 All aqueous solutions contain H+ and OH- ions due to the partial dissociation of water.
- 🔍 An acidic solution has a higher concentration of H+ ions compared to OH- ions, while an alkaline solution has the opposite.
- ⚖️ A neutral solution has equal concentrations of H+ and OH- ions, which is the case for pure water at 298 Kelvin.
- 📈 The ionic product of water (kW) is a constant that represents the product of the concentrations of H+ and OH- ions at equilibrium.
- 🌡️ The value of kW is affected by temperature, with higher temperatures leading to an increase in the dissociation of water and thus a higher kW value.
- 🔥 The dissociation of water is an endothermic process, meaning it absorbs heat.
- 📊 At 298 Kelvin, the pH of pure water is 7 because the concentration of H+ ions is 1 x 10^-7 mol/L.
- 🌡️ Increasing the temperature to 310 Kelvin (321 Kelvin) results in a pH of 6.7 for pure water, showing that the pH decreases with temperature.
- 🤔 Despite changes in pH with temperature, water remains neutral as long as the concentrations of H+ and OH- ions are equal.
- 🧪 The concept of ionic product of water (kW) is essential for understanding the acid-base balance in aqueous solutions.
- 📚 The equilibrium constant expression for the dissociation of water can be used to calculate the concentrations of H+ and OH- ions at any given temperature.
Q & A
What is the ionic product of water, and what is it commonly denoted by?
-The ionic product of water is a constant that represents the product of the concentrations of hydrogen ions (H+) and hydroxide ions (OH-) in an aqueous solution at equilibrium. It is commonly denoted by the symbol kW.
In an aqueous solution, what ions are always present due to the partial dissociation of water?
-In an aqueous solution, hydrogen ions (H+) and hydroxide ions (OH-) are always present due to the partial dissociation of water.
How does the acidity or alkalinity of a solution relate to the concentrations of H+ and OH- ions?
-For an aqueous solution to be acidic, the concentration of hydrogen ions (H+) must be greater than the concentration of hydroxide ions (OH-). Conversely, for a solution to be alkaline, the hydroxide ion concentration must be greater than the hydrogen ion concentration. If the solution is neutral, the concentrations of these two ions are the same.
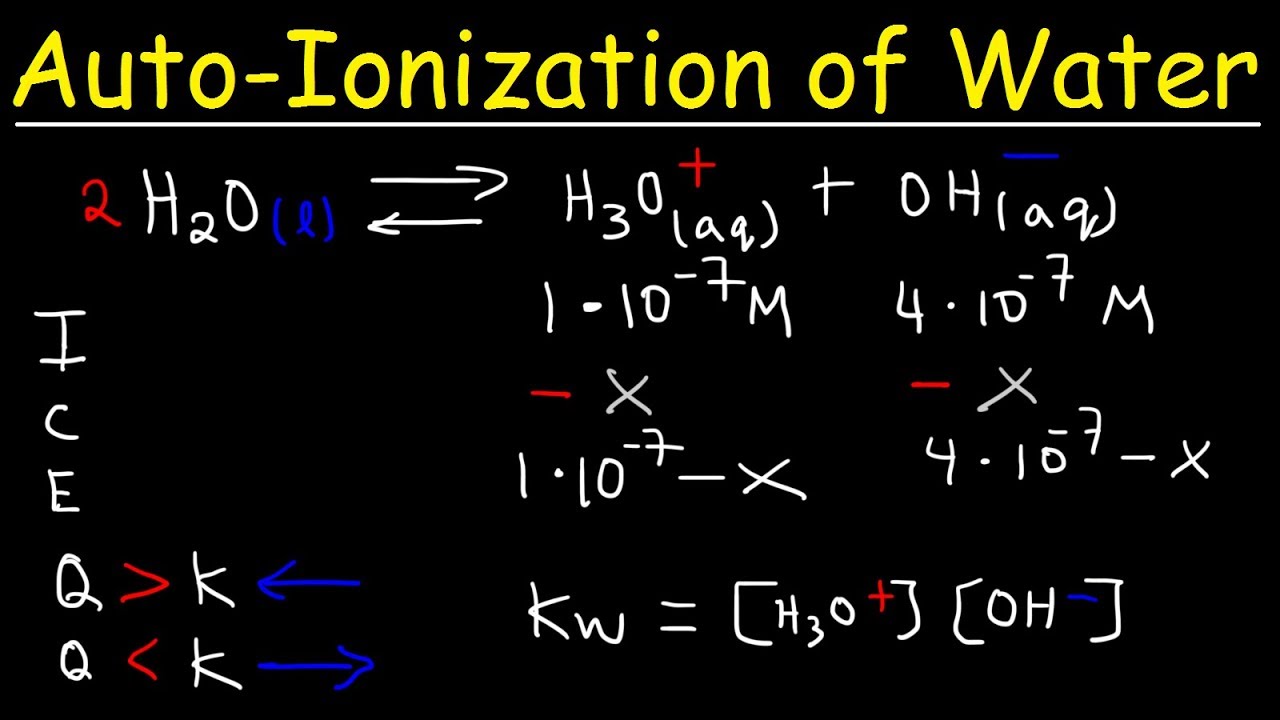
What is the equilibrium constant expression for the partial dissociation of water?
-The equilibrium constant expression for the partial dissociation of water is written as H+ concentration times OH- concentration, or [H+][OH-].
What is the value of kW for water at 298 Kelvin?
-The value of the ionic product of water (kW) at 298 Kelvin is 1 times 10 to the negative 14 mol squared per decimeter cubed (1 x 10^-14 mol^2 dm^-6).
How does temperature affect the ionic product of water (kW)?
-Since the dissociation of water is an endothermic process, an increase in temperature favors the endothermic direction, leading to an increase in the value of kW. This means that as temperature rises, the dissociation of water occurs more readily, resulting in higher concentrations of H+ and OH- ions in the solution.
What is the pH of pure water at 298 Kelvin?
-The pH of pure water at 298 Kelvin is 7, which indicates that it is neutral, as the concentrations of H+ and OH- ions are equal.
How does the pH of water change with temperature?
-As the temperature increases, the pH of water decreases. For example, at 321 Kelvin (which is higher than 298 K), the pH of water is approximately 6.7, which is still considered neutral because the concentrations of H+ and OH- ions remain equal, just at a higher level of dissociation due to the increased temperature.
What is the significance of the ionic product of water (kW) in determining the pH of a solution?
-The ionic product of water (kW) is crucial in determining the pH of a solution because it provides the product of the equilibrium concentrations of H+ and OH- ions. By knowing the value of kW and the concentration of either H+ or OH-, one can calculate the concentration of the other ion and subsequently determine the pH of the solution.
How does the concentration of water molecules affect the calculation of kW?
-The concentration of water molecules is so large compared to the concentrations of H+ and OH- ions that it is effectively considered constant in the calculation of kW. This simplification allows us to focus on the product of the concentrations of the ions rather than the concentration of water itself.
What can be inferred about the dissociation of water at different temperatures based on the value of kW?
-At higher temperatures, the value of kW increases, indicating a higher degree of dissociation of water into H+ and OH- ions. This means that the concentrations of these ions are greater at elevated temperatures, which can affect the pH of the solution.
Outlines
🌊 The Ionic Product of Water (kW) and Its Dependence on Temperature
This paragraph discusses the concept of the ionic product of water, known as kW, which is a result of the partial dissociation of water into H+ and OH- ions. It explains that all aqueous solutions contain these ions due to this process, with a very low dissociation rate of one in every 500 million water molecules. The equilibrium constant for this reaction is introduced, and it is noted that the concentration of water is so high that it can be considered constant, leading to the definition of kW as a new constant. The paragraph further explores how temperature affects the dissociation of water, stating that since the dissociation is endothermic, increasing temperature favors more dissociation, resulting in an increased value of kW. The effect of temperature on the pH of pure water is also discussed, with a calculation showing that at 298 Kelvin, the pH is 7, but at a higher temperature of 310 Kelvin, the pH decreases to 6.7, although water remains neutral as the concentrations of H+ and OH- ions remain equal.
🔥 The Effect of Temperature on the Dissociation of Water and pH
This paragraph delves into the impact of temperature on the dissociation of water and its subsequent effect on the pH level. It emphasizes that the dissociation of water is an endothermic process, meaning that an increase in temperature will shift the equilibrium towards more dissociation, thus increasing the concentration of H+ and OH- ions. This leads to a change in the ionic product of water (kW), which is temperature-dependent. The paragraph provides a clear example by calculating the pH of water at 310 Kelvin, showing that it differs from the pH at 298 Kelvin. Despite the change in pH, the solution remains neutral because the concentrations of hydrogen and hydroxide ions remain equal. This highlights the important principle that the neutrality of a solution is determined by the equalness of these ion concentrations, not by the pH value itself.
Mindmap
Keywords
💡aqueous solutions
💡partial dissociation
💡ionic product of water (Kw)
💡pH
💡hydroxide ion (OH-)
💡equilibrium constant
💡concentration
💡endothermic reaction
💡temperature effect
💡acidic solution
💡alkaline solution
Highlights
All aqueous solutions contain H+ ions and/or OH- ions, resulting from the partial dissociation of water.
In an aqueous solution, the concentration of hydrogen ions (H+) must be greater than the hydroxide ions (OH-) for the solution to be acidic.
For a solution to be alkaline, the hydroxide ion concentration must be greater than the hydrogen ion concentration.
A neutral solution has equal concentrations of H+ and OH- ions.
The equilibrium constant for the dissociation of water is represented as the ionic product of water (kW).
The value of kW at 298 Kelvin is 1 x 10^-14 mol^2/dm^3.
The pH of pure water at 298 Kelvin is 7 due to the equal concentration of H+ and OH- ions.
The dissociation of water is an endothermic process, meaning it absorbs heat.
Increasing temperature favors the endothermic direction, causing more dissociation of water.
As temperature increases, the value of kW increases.
At 310 Kelvin, the pH of water is approximately 6.7, still considered neutral as the concentrations of H+ and OH- remain equal.
The concentration of water is treated as constant in the dissociation equilibrium due to its large value compared to H+ and OH-.
The equilibrium expression for water dissociation is written as kW = [H+][OH-].
The ionic product of water (kW) is a new constant derived from the product of two constants.
The pH scale is used to measure the acidity or alkalinity of a solution based on the concentration of H+ ions.
The dissociation of water is represented by the equation H2O ↔ H+ + OH-.
The equilibrium constant expression for water dissociation is simplified to [H+]^2 = kW at 298 Kelvin.
The concentration of H+ in pure water at 298 Kelvin is 1 x 10^-7 mol/dm^3, leading to a pH of 7.
The pH of water changes with temperature, but the solution remains neutral as long as the concentrations of H+ and OH- are equal.
The value of kW at 310 Kelvin is 4 x 10^-14 mol^2/dm^3, resulting in a pH of 6.7 for pure water.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: